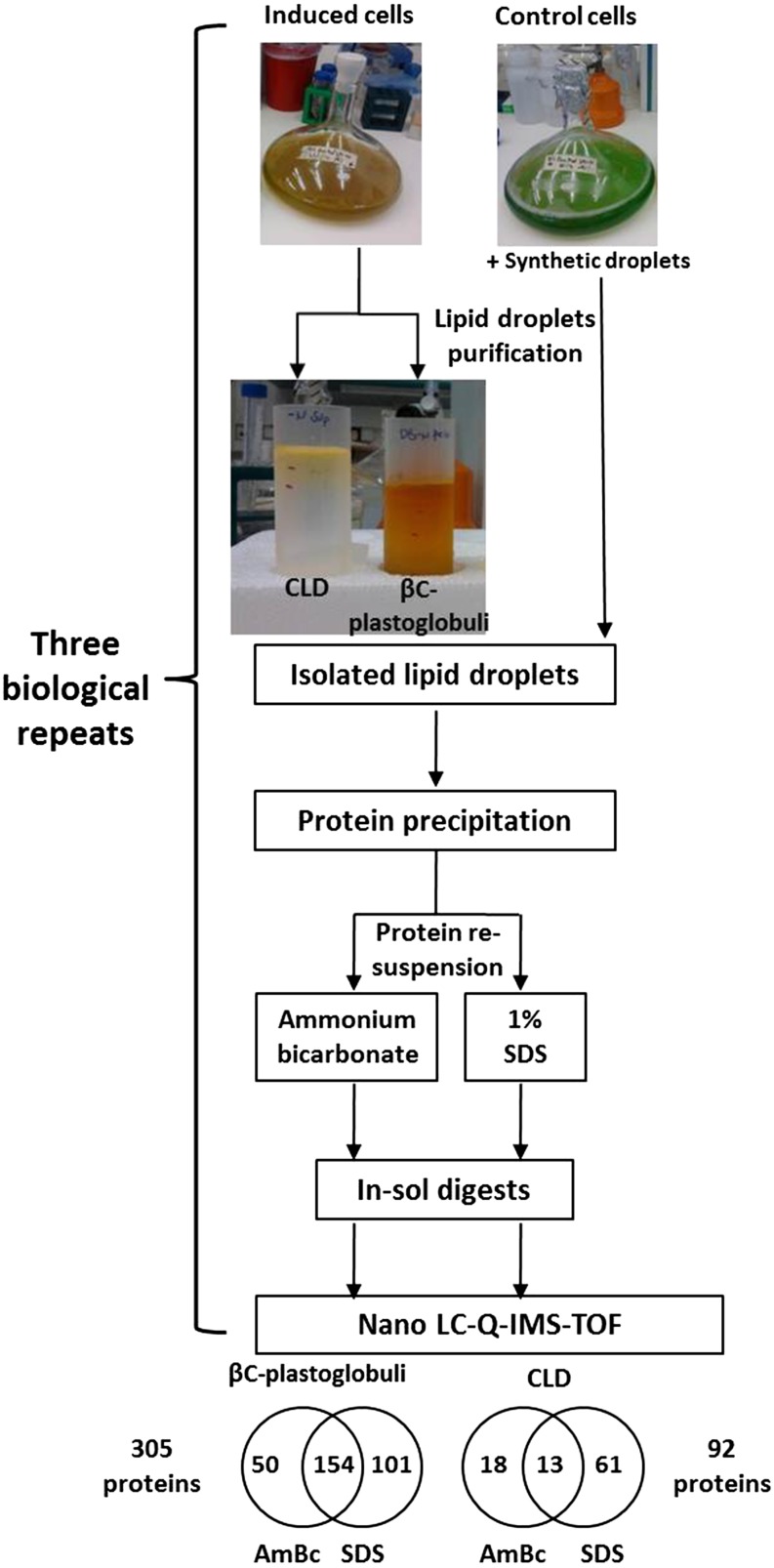
Figure 1.
Scheme of the isolation and identification of the CLD and βC-plastoglobuli proteomes. Lipid droplets were prepared from D. bardawil cells cultured without nitrogen for 2 d and from nitrogen-sufficient cells supplemented with synthetic globules. Thylakoids were isolated from nitrogen-sufficient cells. Proteins were precipitated in acetone and resuspend first in 50 mm AmBc and next in 1% SDS. Each sample was digested with trypsin. Peptide digests were analyzed by nanoliquid chromatography quadrupole ion mobility time-of-flight mass spectrometry (LC-Q-IMS-TOF). A total of 570 proteins were identified in both lipid pools. Proteins with at least two peptides in at least two biological repeats were analyzed. A total of 305 and 92 unique proteins were identified in βC-plastoglobuli and CLD, respectively, 154 and 13 of which were identified by both experimental methods in βC-plastoglobuli and CLD, respectively. In-sol, In solution.