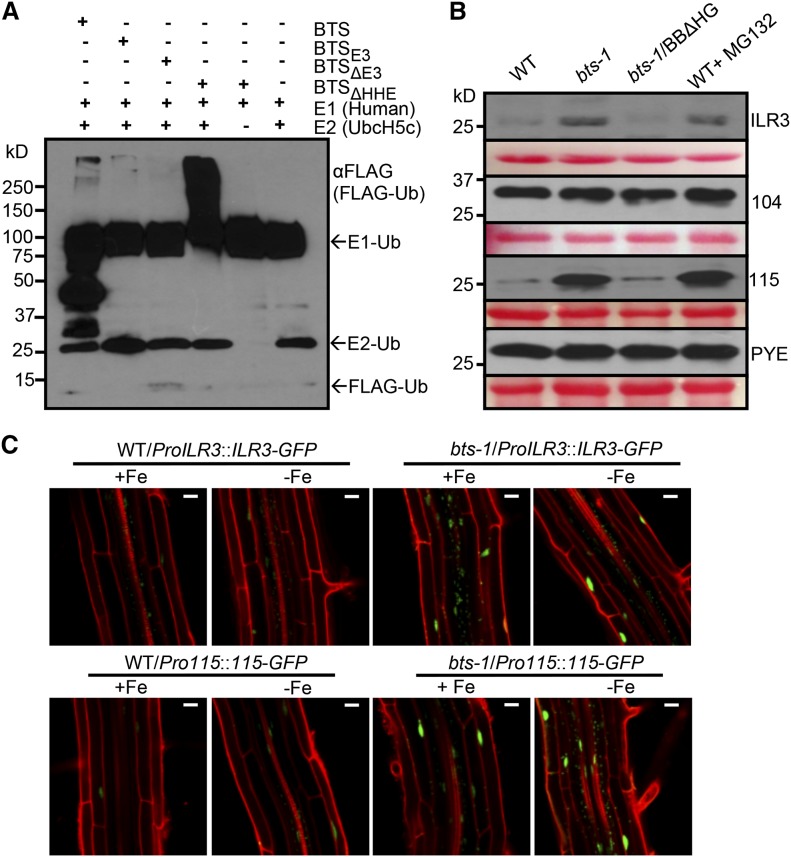
Figure 5.
BTS targets ILR3 and bHLH115 for 26S proteasome-mediated degradation. A, E3 ligase activity of BTS and its derivatives. In vitro-translated and purified 3xHA-tagged BTS, BTSE3 domain, and BTSΔE3 and BTSΔHHE deletion proteins were subjected to in vitro ubiquitination assay containing ATP, FLAG-tagged ubiquitin (Ub), and human E1 and E2 (UbcH5c). Proteins were immunodetected using anti-FLAG antibody. Results shown represent two independent assays. B, Cell-free 26S proteasome-mediated degradation of PYEL proteins by BTS and BTSΔHHE. In vitro-translated and purified Myc-tagged ILR3, bHLH104, bHLH115, and PYE proteins were incubated with cell-free protein extracts prepared from 7-d-old seedlings of the wild type (WT), bts-1, and bts-1/ProBTS::BTSΔHHE-GFP (BBΔHG) grown on –Fe medium (4 d +Fe and 3 d –Fe). Reactions were performed with and without 160 µm MG132 (proteasome inhibitor). Proteins were immunodetected using anti-Myc antibody. Ponceau S staining indicates equal amount of cell-free protein extract loaded. C, Stability of ILR3-GFP and bHLH115-GFP in wild-type and bts-1 seedlings expressing either ProILR3::ILR3-GFP (top) or Pro-115::115-GFP (bottom) in roots. Plants were grown for 6 d on ±Fe medium (4 d +Fe and 2 d ±Fe). Results shown represent two independent assays. Bars = 20 μm.