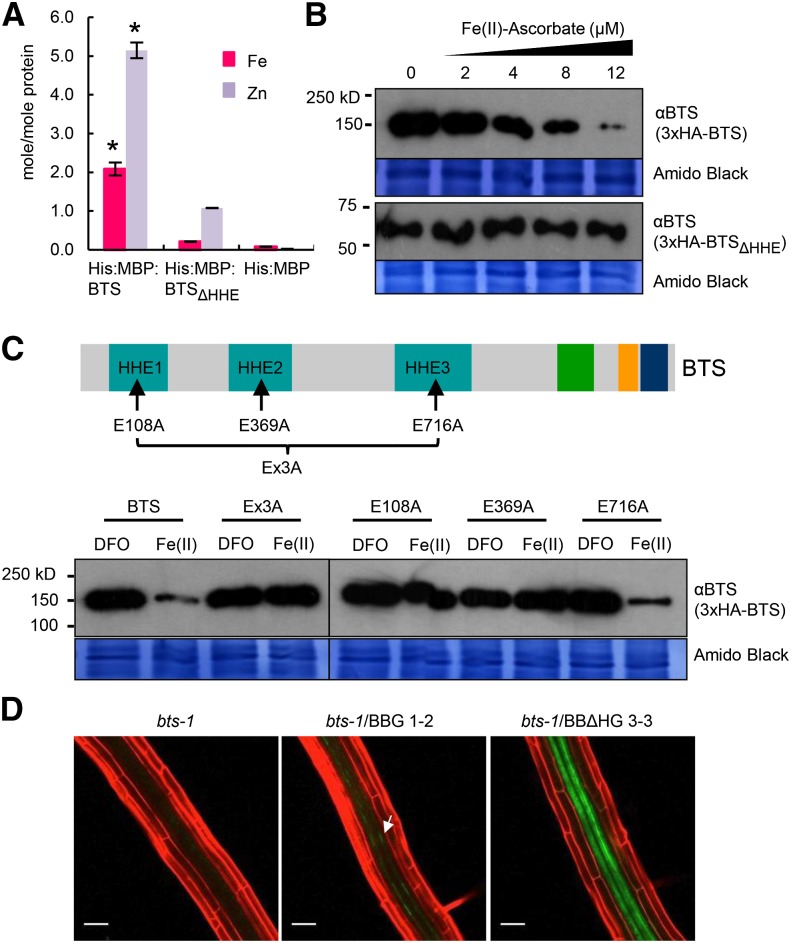
Figure 6.
BTS binds iron through HHE domains, negatively affecting BTS protein stability. A, Iron and zinc content of His:MBP fusions of BTS and HHE deleted (BTSΔHHE) proteins or His:MBP expressed in E. coli and purified. Error bars indicate ± se of the mean (n = 3 for His:MBP and His:MBP:BTS and n = 2 for His:MBP:BTSΔHHE). Asterisks indicate significant difference from BTSΔHHE and His:MBP (P < 0.05). B, In vitro translation of 3xHemagglutinin (3xHA)-tagged BTS and BTSΔHHE proteins performed in the presence of increasing concentration of ferrous iron (Fe(II)-ascorbate). Proteins were immunodetected using anti-BTS antibody. Amido Black staining indicates equal amount of wheat germ extract were loaded from in vitro protein translations. Results shown represent two independent assays. C, In vitro translation of 3xHA-tagged wild-type BTS, single amino acid substitutions (E108A, E369A, and E716A), and triple amino acid substitution (Ex3A) BTS proteins were performed in presence of ferrous iron chelator (100 μm DFO) and ferrous iron [12 μm Fe(II)-ascorbate]. Proteins were immunodetected using anti-BTS antibody. Amido Black staining indicates that equal amounts of wheat germ extract were loaded from in vitro protein translations. Results shown represent two independent assays. D, In planta stability of BTSΔHHE protein. Confocal microscopy images of roots of 7-d-old (4 d +Fe and 3 d –Fe) bts-1 seedlings expressing ProBTS::BTS-GFP (BBG, arrows) and ProBTS::BTSΔHHE-GFP (BBΔHG) stained with propidium iodide (red). Results shown represent four independent assays. Scale bars = 50 μm.