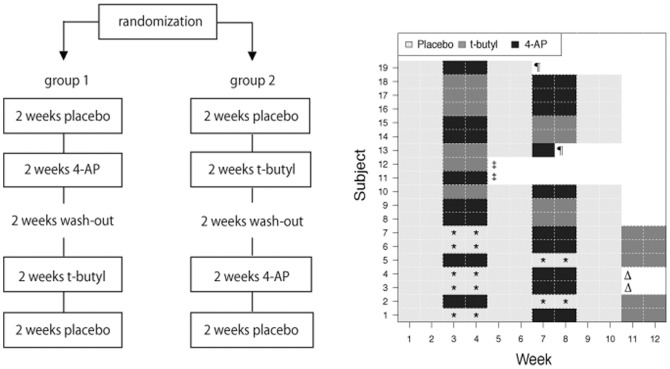
Figure 1. Study design and patient treatment allocation.
A: Diagram of the study design. B: Treatment assignments for each subject by week. Boxes indicated by an asterisk (*) represent sub-therapeutic dosing with t-butyl. These and the preceding two-week treatment periods were removed from statistical analysis. Symbols indicate dogs that did not complete the trial; Δ: owners unable to continue; ‡: euthanasia; ¶: discontinuation due to adverse effects. Dog 13 received just 2 doses of 4-AP prior to development of seizures. Dog 19 received 10 doses of 4-AP prior to developing gastrointestinal signs.