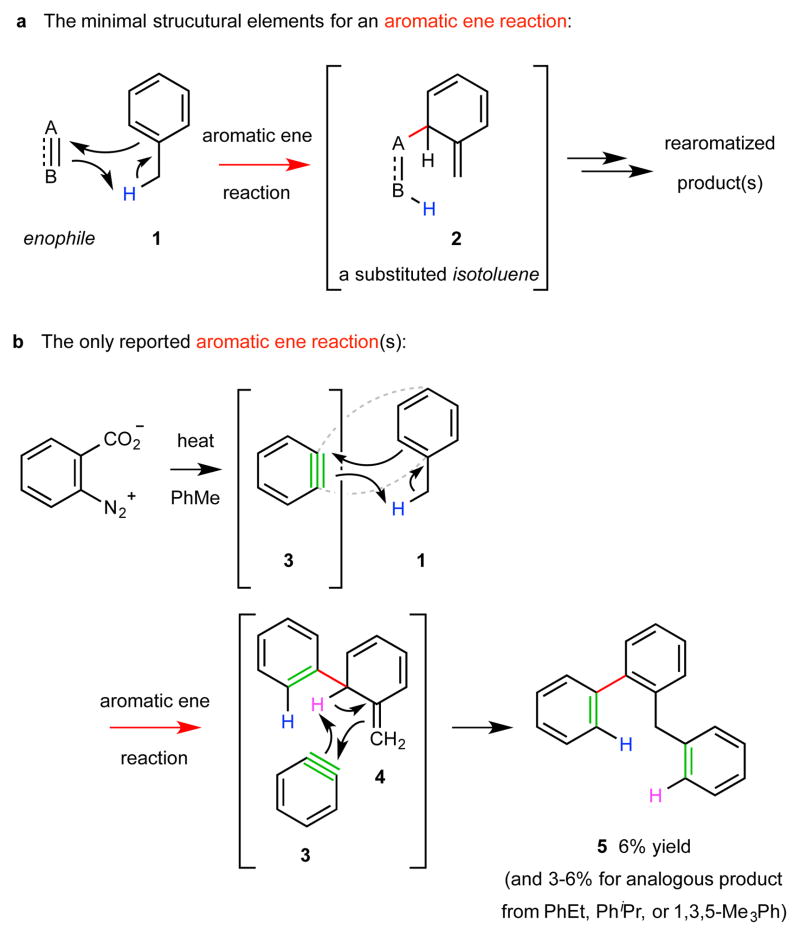
Figure 1. Structural delineation and previous example of an aromatic ene reaction.
a, The minimal structural elements for an aromatic ene reaction: a potent enophile and an arene bearing a benzylic C–H bond as an ene donor. This transformation is rare because it requires the energetically demanding formation of a dearomatized isotoluene species (cf. 2). b, The only reported aromatic ene reactions involve the use of the highly energetic o-benzyne (3) in the role of enophile, which engages one of the alkylbenzenes toluene (1), ethylbenzene, cumene, or mesitylene as the ene donor. The intermediate isotoluene 4 was invoked to account for formation of product 5. Yields are marginal in part because products arising from [4+2] cycloaddition between 3 and the π-system in arene 1 are formed competitively.