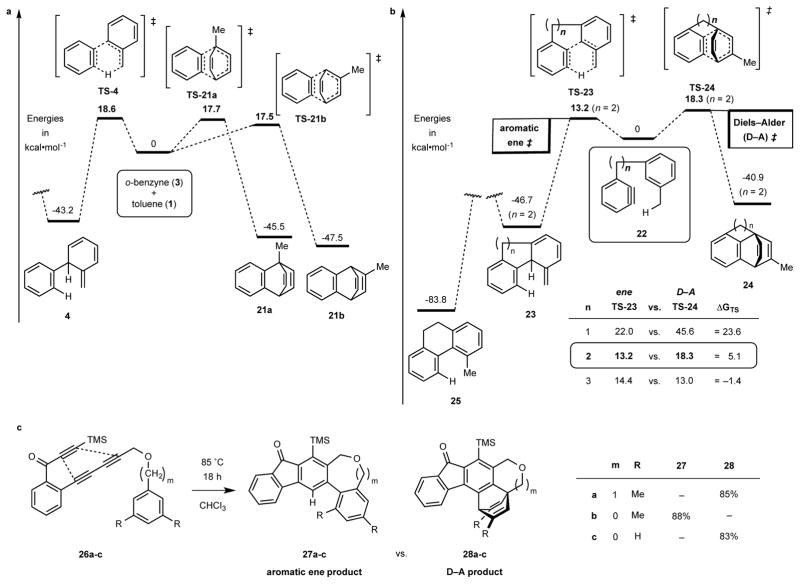
Figure 3. Competition between aromatic ene and aromatic Diels-Alder pathways.
a, Computed (DFT) energetics of possibilities for the bimolecular reaction between toluene (1) and o-benzyne (3) leading to the aromatic ene product 4 (an isotoluene) vs. the Diels-Alder adducts 21a and 21b. b, Computed energetics of the analogous competitions for the tethered substrates 22 leading to the intramolecular ene product 23 vs. the [4+2] adduct 24; the potential energy surface is portrayed for the case where n = 2 and the comparative values of the ΔGTS are tabulated in the inset. c, Experimental results for the series of related HDDA substrates 26a-c; the first and last give, exclusively, the Diels–Alder products 28a and 28c whereas 26b gives only the aromatic ene product 27b.