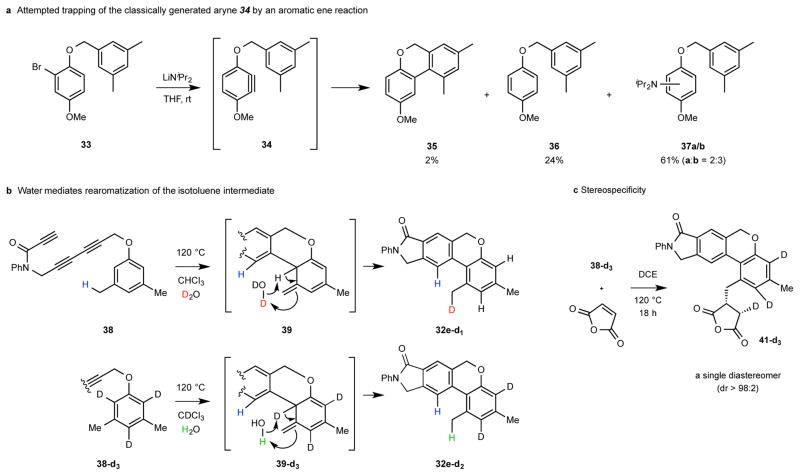
Figure 4. Studies giving insight to a) the importance of reaction conditions and b/c) the mechanism of the HDDA//aromatic ene reactions.
a, Attempted aromatic ene reaction of the aryne 34 generated by elimination of HBr from the aryl bromide 33 shows an advantage of use of the reagent-free conditions of the HDDA reaction as the method for aryne formation. b, A pair of complementary deuterium-labeling experiments strongly implicate the intermediacy of the isotoluene 39. c, The cis-addition of carbon and deuterium across the maleic anhydride π-bond (see Supplementary Information for a discussion of the 1H NMR-based assignment of the structure 41-d3) provides strong mechanistic evidence for the concerted nature of the bimolecular (Alder) ene reaction.