Abstract
Necrotic cell death in vivo induces a robust neutrophilic inflammatory response and the resulting inflammation can cause further tissue damage and disease. Dying cells induce this inflammation by releasing pro-inflammatory intracellular components, one of which is uric acid. Cells contain high levels of intracellular uric acid, which is produced when purines are oxidized by the enzyme xanthine oxidase. Here we test whether a non-nucleoside xanthine oxidase inhibitor, Febuxostat (FBX), can reduce intracellular uric acid levels and inhibit cell death-induced inflammation in two different murine tissue injury models; acid-induced acute lung injury and acetaminophen liver injury. Infiltration of inflammatory cells induced by acid injection into lungs or peritoneal administration of acetaminophen was evaluated by quantification with flow cytometry and tissue myeloperoxidase activity in the presence or absence of FBX treatment. Uric acid levels in serum and tissue were measured before giving the stimuli and during inflammation. The impact of FBX treatment on the peritoneal inflammation caused by the microbial stimulus, zymosan, was also analyzed to see whether FBX had a broad anti-inflammatory effect. We found that FBX reduced uric acid levels in acid-injured lung tissue and inhibited acute pulmonary inflammation triggered by lung injury. Similarly, FBX reduced uric acid levels in the liver and inhibited inflammation in response to acetaminophen-induced hepatic injury. In contrast, FBX did not reduce inflammation to zymosan, and therefore is not acting as a general anti-inflammatory agent. These results point to the potential of using agents like FBX to treat cell death-induced inflammation.
Keywords: febuxostat, inflammation, uric acid, acetaminophen, lung damage, liverdamage
1. Introduction
Inflammation is a host response to injury or infection where elements of the innate immune response are rapidly mobilized to the affected site (Majno and Joris, 2004). Soluble immune components such as complement and collectins are delivered into the tissue through leaking vessels and neutrophils and macrophages migrate into the tissue site. These innate defenses then attempt to eliminate or contain the injurious process.
When cells die in vivo, particularly by necrosis, they invariably trigger an acute inflammatory response (Rock et al., 2011). Within hours the tissue becomes infiltrated with leukocytes, first neutrophils and then macrophages. This response is so stereotypical that its progression can be used to date the time of cell injury. This inflammatory response is seen even in situations where the injury is sterile, e.g. induced by ischemia or toxic agents (Rock et al., 2011). In many of these situations, the acute inflammatory response is not effective in limiting the cell injury and may actually make it worse (Chen and Nunez, 2010; Kono and Rock, 2008; Rock et al., 2011). This is because many of the immune cells’ effector molecules (e.g. reactive oxygen species, proteases, etc.) can damage normal tissues when they leak from living and dying leukocytes. This pathological effect is seen e.g. in ischemia-reperfusion injury and drug-induced tissue injury (Jaeschke et al., 2012; Kurts et al., 2013). Since sterile inflammation can contribute to disease pathogenesis, it is a potential therapeutic target.
There has been considerable progress in understanding how dying cells trigger a sterile immune response. When cells die by necrosis they lose integrity of their plasma membrane and their intracellular molecules leak into the extracellular fluids. The innate immune system has evolved mechanisms to detect the release of a subset of these molecules, call DAMPs (damage-associated molecular patterns) (Rock et al., 2010). When macrophages sense the presence of DAMPs they are stimulated to produce pro-inflammatory cytokines that then induce inflammation (Kono et al., 2010b). The cytokine IL-1 is particularly important for acute neutrophilic inflammation (Chen et al., 2007).
One of the important DAMPs is uric acid (Kono et al., 2010a; Shi et al., 2003). Uric acid was discovered over 100 years ago to be the etiologic agent that incites inflammation in the disease gout. In this condition, patients with hyperuricemia nucleate crystals of monosodium urate in their joint or elsewhere that then triggers inflammation (Doherty et al., 2012; Neogi, 2011; Richette and Bardin, 2010; Rock et al., 2013). More recently, it has been shown that in normal individuals uric acid release from dying cells acts locally to cause acute inflammation and stimulate dendritic cells (Kono et al., 2010a; Shi et al., 2003). Massive cell death can raise levels of uric acid systemically and cause systemic inflammation, e.g. in tumor lysis syndrome (Howard et al., 2011).
Uric acid is generated when cells break down their purines (Rock et al., 2013). In this process the cellular peroxisomal enzyme xanthine oxidase, converts the purines into uric acid through an oxidation reaction. This process occurs continuously in cells, resulting in constitutively high intracellular levels of uric acid that can further increase when cells are injured or die and degrade their RNA and DNA (Kono et al., 2010a). Inhibitors of xanthine oxidase have been developed and are currently used clinically to reduce serum levels of uric acid and prevent flares of disease in gout patients (Neogi, 2011). In this report we investigate whether the non-nucleoside xanthine oxidase inhibitor Febuxostat can reduce uric acid levels in tissues and inhibit inflammation to tissue injury.
2. Materials and Methods
2.1. Mice
C57BL/6 male mice were purchased from Jackson Laboratory and bred in our vivarium. 8–13 week old male mice were used in all experiments. 2–5 mice per group were used in individual experiments and experiments were repeated multiple times as indicated in figure legends. The total number (n) of animals per experimental condition is indicated in the figures. All animal experiment protocols were approved by the University of Massachusetts Medical School Animal Care and Use Committee.
2.2. Materials
Febuxostat powder (FBX; PubChem CID:134018) was provided by Takeda USA. To prepare Febuxostat-containing drinking water for five mice, 4 or 8 mg of Febuxostat powder dissolved in 0.05N NaOH was added into 250ml water after sonication for 30min. The pH was adjusted to 7.2 by adding 0.1N HCl and the final concentration of Febuxostat was 0.027mg/ml after adding water to 300ml in total volume so as to give 5 or 10mg/kg/day Febuxostat per mouse. Febuxostat treatment was continuous throughout the experiment. Allopurinol (ALP)(UDL Laboratories, Morgantown, WV) was dissolved in drinking water and administered at a concentration of 0.1mg/ml (20mg/kg/day).
7/4 FITC and 7/4 Alexa 647 antibodies were purchased from AD serotec. Ly6G PE antibody was purchased from BD. CD11b PerCP5.5 and F4/80 APC antibodies were bought from e-Bioscience. 7-aminoactinomycin D (7AAD) was purchased from Life technologies.
2.3. Uric acid measurement
Uric acid level was measured by using QuantiChrom Uric Acid Assay Kit (BioAssay Systems) in serum samples according to the manufacturer’s protocol. Lungs and livers were homogenized in the PBS buffer including 1% Triton-X and 0.1% SDS at 200mg/ml. Homogenates were passed through 3K Amicon Ultra Filters (Millipore) and the filtrates were applied for measuring uric acid. The OD595nm value was analyzed on the plate reader (iMark microplate reader (BioRad)).
2.4. Acid-induced acute lung injury
FBX- or ALP-containing drinking water was initiated 3 days before challenging hydrochloric acid buffered in 0.02M citric acid (pH1.8). Each mouse was anesthetized with 100mg/kg ketamine plus 10mg/kg xylazine by intraperitoneal injection and then given 75μl acid intratracheally preferentially into the left lung by tilting the body to the left side while and after injection to reduce the possibility of drowning, or no injection. Bronchial alveolar lavage (BAL) fluid was collected by 2 intratracheal injections of 700μl recovery media containing 2% fetal bovine serum in RPMI media with 3mM EDTA and 10 units/ml heparin at indicated time points and both lungs were resected after perfusion with 30ml PBS and stored at −80 °C until uric acid level and myeloperoxidase activity were measured. Infiltrating cells in BAL samples were pelleted by centrifugation at 500 g for 5min and stained with Ly6G PE, 7/4 Alexa647, 7AAD and 2.4G2 at 1:50 dilution for 30 min at 4 °C. The number of BAL neutrophils (Ly6G+7/4+) and macrophages (Ly6G-/low7/4+) was quantified with flow cytometry by co-counting 25000 of 15μm microsphere beads (Polysciences Inc.) mixed in each sample.
2.5. Acetaminophen-induced liver injury
Acetaminophen (Sigma) was dissolved at the concentration of 15mg/ml in PBS heated at 55 °C. After an overnight fast, 300mg/kg acetaminophen solution was administrated intraperitoneally and the treated mice were provided food 4 h later. After 18 h from acetaminophen administration, the mice were humanely killed and their livers perfused with 30 ml HBSS buffer introduced through the inferior vena cava. The livers were then treated with a buffer containing 0.05% collagenase IV (Sigma), 0.028% DNase I (Sigma), 1.25 mM CaCl2 and 4 mM MgCl2 in HBSS buffer (Gibco) at 37 °C. Nonparenchymal cells were isolated from whole liver cells in 50% OptiPrep density gradient medium (Sigma) diluted with RPMI media and stained with 7/4 FITC, Ly6G PE, CD11b Cy5.5 and F4/80 APC antibodies at 1:100 concentration each in 2.4G2 supernatant. Recruited inflammatory cells were counted on BD High Throughput Sampler-installed FACSCalibur (BD).
2.6. Myeloperoxidase (MPO) assay
Tissue homogenate of lungs or livers was made with a TissueLyzer II (QIAGEN) in MPO buffer containing 50mM Na2HPO4, 0.5% Hexadecyltrimethyl Ammonium Bromide and 10mM EDTA (pH5.4) at the concentration of 200mg/ml. After 10-min centrifugation at 16000 g, supernatant of lung homogenate or sonicated liver homogenate was collected and assayed for MPO activity by adding 16.7mg/ml O-dianisidine and the developer 0.25 wt % H2O2 and then following the kinetic change of 450nm OD value was measured in an iMark microplate reader (BioRad) at 15 s intervals between 0 min and 2 min 20 s.
2.7. Zymosan-induced peritonitis
Zymosan A from Saccharomyces cerevisiae (Sigma) was suspended in PBS at 0.5mg/ml and 100μg zymosan solution was intraperitoneally injected into Febuxostat treated or control mice. To harvest peritoneal inflammatory cells, mice were humanly killed and 10ml of recovery media which is RPMI containing 2% fetal bovine serum, 10U/ml heparin and 3mM EDTA was injected into and recovered from the peritoneal cavity of each mouse. Infiltrating cells in 500μl lavage fluid were pelleted and stained in the same way as the lung injury experiment and then counted on BD High Throughput Sampler-installed FACSCalibur.
2.8. Statistics
Data are reported as means ± standard deviations (S.D.) and sample numbers are also indicated. Data in each arm of all the independent experiments was judged by D’Agostino and Pearson omnibus normality test to determine whether the distribution was normal. Statistical analysis in each experiment was performed by one-way ANOVA if the data distributed normally, otherwise the Mann-Whitney U test was used for the analysis. All the statistical calculations were made by Prism software version 6 (GraphPad Software). P value was considered significant if it is less than 0.05.
3. Results
3.1. Effect of Febuxostat on levels of uric acid in lung and liver
Febuxostat is orally bioavailable and is active against both human and mouse xanthine oxidase. When this agent is administered in vivo, it is known to reduce the levels of uric acid in serum. However, the drug’s effect on intracellular levels of uric acid in tissues has not been reported. We focused on this issue for lung and liver, because the release of intracellular uric acid has been implicated in the inflammation that develops in both these organs after injury. To investigate this issue, we administered Febuxostat to mice in their drinking water at therapeutic doses for 3 days. We then harvested serum, lungs and livers from control or Febuxostat-treated mice and measured their content of uric acid.
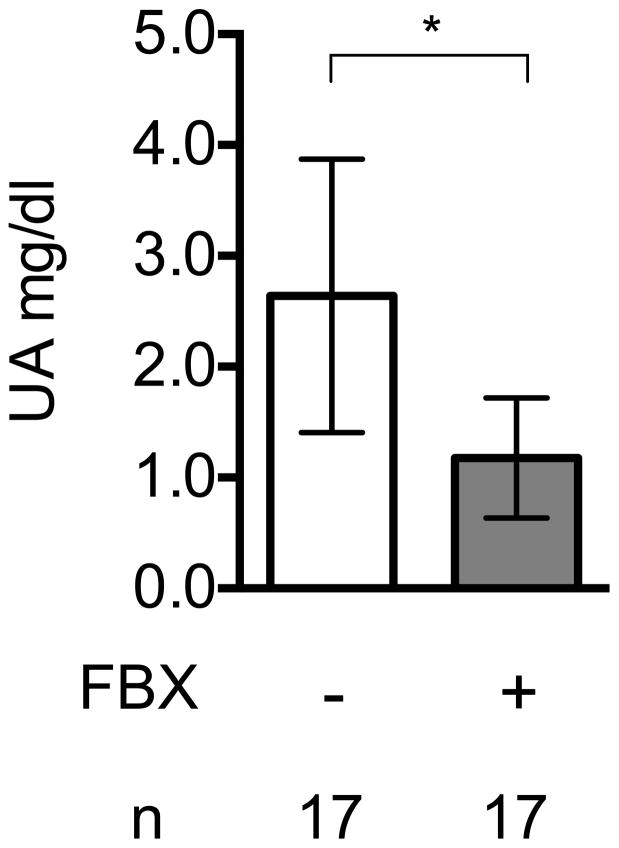
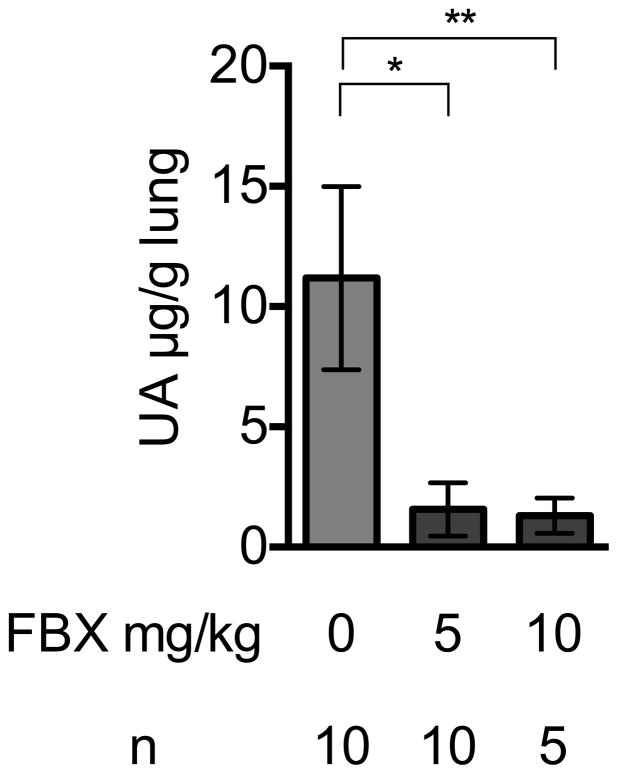
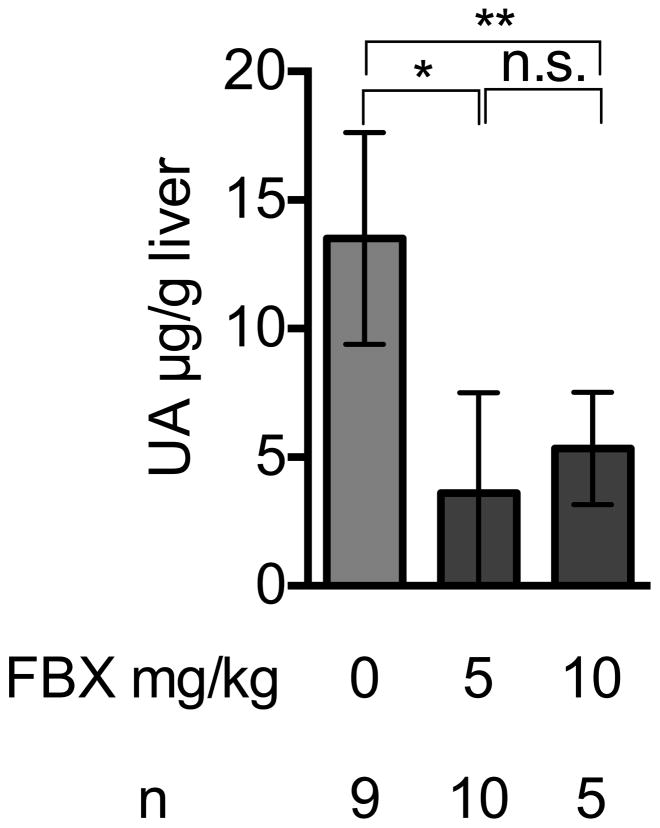
Febuxostat reduced the levels of uric acid in the serum of treated mice, as expected (Fig 1A). The mean uric acid levels in lung and liver were also reduced in mice treated with Febuxostat at two different doses, 5mg/kg and 10mg/kg (Fig 1B, 1C). The higher dose of febuxostat reduced uric acid in serum (supplementary data 1) and lung tissue (Fig 1B) slightly better than lower dose (5mg/kg), although this difference was not statistically significant. Therefore, we used 10mg/kg febuxostat in all subsequent experiments.
Fig. 1. Febuxostat lowers basic uric acid level in lung and liver.
Fig. 1A
Uric acid lowering effect of Febuxostat
Uric acid was measured in serum of control or Febuxostat (FBX)-treated mice. Means and standard deviations (S.D.) were calculated from the accumulated data of 4 independent experiments. In individual experiments the number of mice per condition was 3–5 for experimental groups and 3–5 in the untreated controls. The bar shows the mean of the data and the limiting bars show the standard deviation. (n = total number of mice from the multiple experiments for each group). * P<0.01 (Mann-Whitney U test)
Fig. 1B
Effect of Febuxostat on Uric acid levels in lung homogenates
Uric acid was measured in the whole left lung of control or, 5 or 10mg/kg Febuxostat (FBX)-treated mice. Means and S.D. were calculated from the accumulated data of 2 independent experiments. In individual experiments the number of mice per condition was 5 for experimental groups and 2–3 in the untreated controls. The bar shows the mean of the data and the limiting bars show the standard deviation. (n = total number of mice from the multiple experiments for each group). *,**P<0.05 (one-way ANOVA)
Fig. 1C
Febuxostat effect on uric acid in liver homogenates
Uric acid was measured in the liver homogenates of control or Febuxostat (FBX)-treated mice Means and S.D. were calculated from the accumulated data of 2 independent experiments. In individual experiments the number of mice per condition was 4–5 for experimental groups and 2–3 in the untreated controls. The bar shows the mean of the data and the limiting bars show the standard deviation. (n = total number of mice from the multiple experiments for each group). *,** P<0.05 (one-way ANOVA)
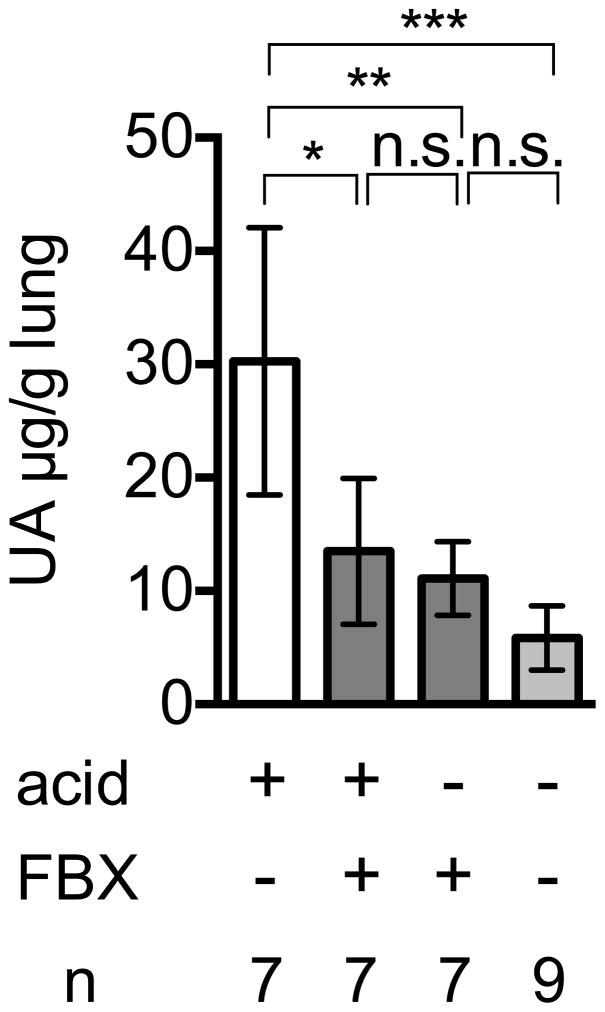
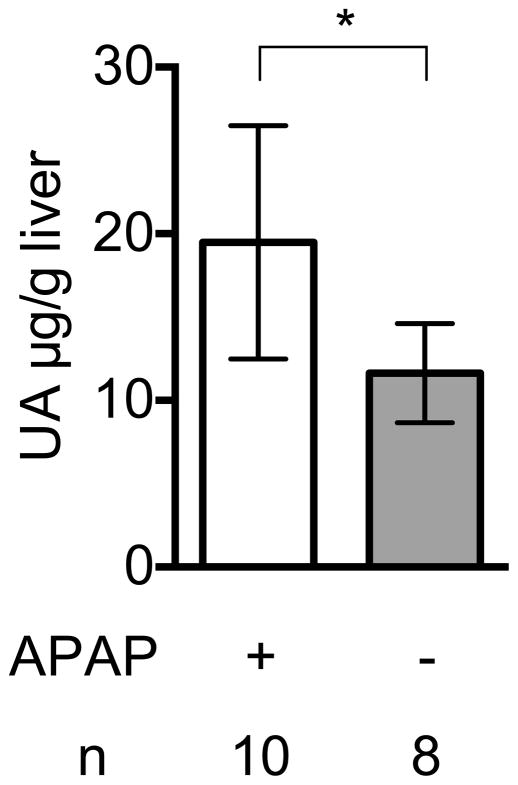
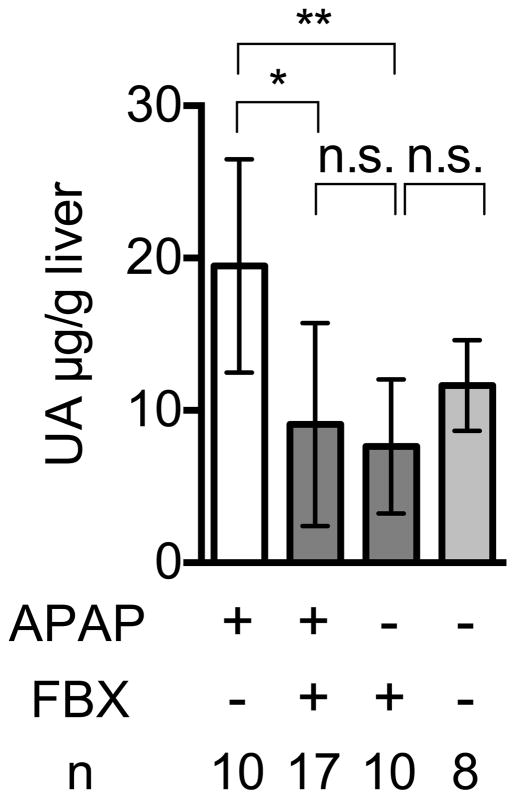
We have previously reported that levels of uric acid markedly increase when cultured cells were injured and catabolize their RNA and DNA (Shi et al., 2003). Here we found a similar increase in uric acid levels after acid-induced injury of the lung (Fig. 2A) and acetaminophen-induced injury of the liver (Fig 2B); these models are described in more detail below. We further found that Febuxostat treatment markedly inhibited this injury-induced elevation in tissue uric acid levels (Fig 2A, 2C).
Fig. 2. Febuxostat inhibits elevation of uric acid in acute pulmonary inflammation induced by intratracheal acid challenge and in acetaminophen-induced acute liver damage.
Fig. 2A
Uric acid in lung homogenates after acid treatment
C57BL/6 mice were treated with or without Febuxostat (FBX) in their drinking water for 3 days before acid injection. The mice were then given 75μl acid intratracheally and uric acid in the whole left lung was measured 6 h post acid administration. Means and S.D. were calculated from the accumulated data of 3 independent experiments. In individual experiments the number of mice per condition was 2–3 for experimental groups and 3 in the untreated controls. The bar shows the mean of the data and the limiting bars show the standard deviation. (n = total number of mice from the multiple experiments for each group). *, **, *** P<0.05, n.s.; not significant (Mann-Whitney U test)
Fig. 2B
Uric acid in damaged livers by acetaminophen
Uric acid was measured in the liver homogenates of control or 300mg/kg acetaminophen-treated mice for 18 h. Livers were perfused with 30ml HBSS buffer before homogenization. Means and S.D. were calculated from the accumulated data of 2 independent experiments. In individual experiments the number of mice per condition was 5 for experimental groups and 4 in the untreated controls. The bar shows the mean of the data and the limiting bars show the standard deviation. (n = total number of mice from the multiple experiments for each group). * P<0.01 (unpaired two-tailed t-test)
Fig. 2C
Effect of Febuxostat on uric acid in acetaminophen-injured livers
Uric acid in acetaminophen-injured liver homogenates was analyzed as in 2b. Means and S.D. were calculated from accumulated data of 4 independent experiments. In individual experiments the number of mice per condition was 2–5 for experimental groups and 2 in the untreated controls. The bar shows the mean of the data and the limiting bars show the standard deviation. (n = total number of mice from the multiple experiments for each group). *,** P<0.05, n.s.; not significant (one-way ANOVA)
3.2. Effect of Febuxostat on the neutrophilic inflammation to acid-induced lung injury
The aspiration of gastric acid into the lungs injures cells and induces inflammation. The acid-induced injury and inflammation can cause cough, asthma, bronchitis, or pneumonia and lead to pulmonary fibrosis. Clinically this is a significant problem in some patients with gastro-esophageal reflux and is also a problem in other settings with aspiration of gastric contents. These effects can be investigated in a mouse model where dilute hydrochloric acid (pH.1.20) is instilled into the lung.
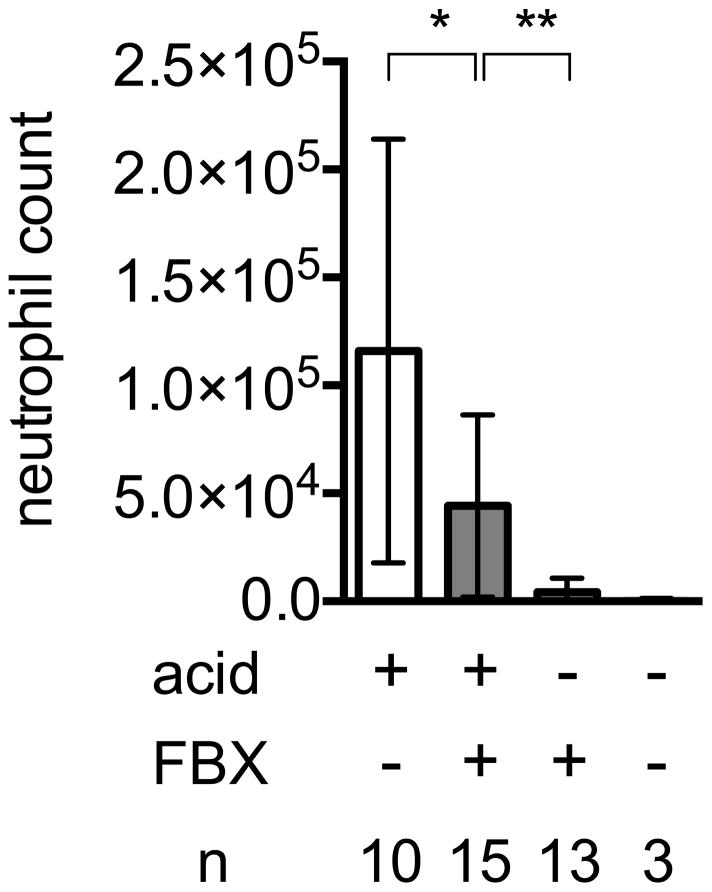
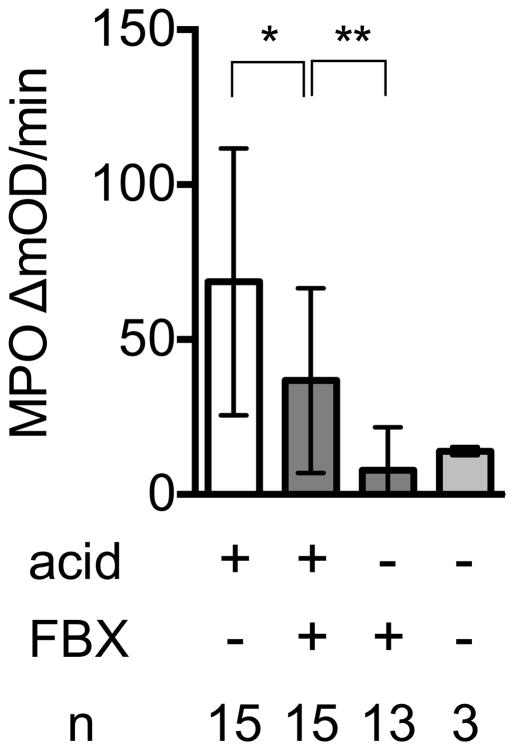
To investigate the effect of Febuxostat on the inflammation arising from cell injury in the lung, we instilled acid into the lungs of either control or Febuxostat-treated mice, and 6 h later, neutrophilic inflammation in the tissues was measured by quantifying the number of neutrophils in bronchial lavage fluid by flow cytometry and also by measuring the PBS-perfused tissue content of a neutrophil-specific enzyme, myeloperoxidase. In normal (nonacid-treated) lungs of mice, there were few neutrophils present. In contrast in the lungs of acid-treated control mice there was a significant accumulation of neutrophils (Fig 3A). In mice treated with Febuxostat there was a significant reduction in the neutrophilic inflammation in response to the acid injury (Fig 3A, B). Allopurinol, a structurally distinct xanthine oxidase inhibitor, also reduced neutrophil infiltration into acid challenged lungs as much as febuxostat, supporting the concept that inhibition of xanthine oxidase reduced injury-induced inflammation (supplementary data 2).
Fig. 3. Uric acid involvement in acid-induced acute pulmonary inflammation.
Fig. 3A
Effect of Febuxostat on neutrophil infiltration into lungs in response to intratracheal acid challenge.
C57BL/6 mice were treated with or without febuxostat (FBX) in their drinking water for 3 days before acid injection. The mice were then given 75μl acid intratracheally and bronchial lavage (BAL) was performed 6 h later. The number of infiltrating inflammatory cells in total BAL fluid was counted on flow cytometry by co-analysis of 25000 counting beads. Means and S.D. were calculated from the accumulated data of 4 independent experiments. In individual experiments the number of mice per condition was 2–4 for experimental groups and 1 in the untreated controls. The bar shows the mean of the data and the limiting bars show the standard deviation. (n = total number of mice from the multiple experiments for each group). *P<0.05, **P<0.01 (Mann-Whitney U test)
Fig. 3B
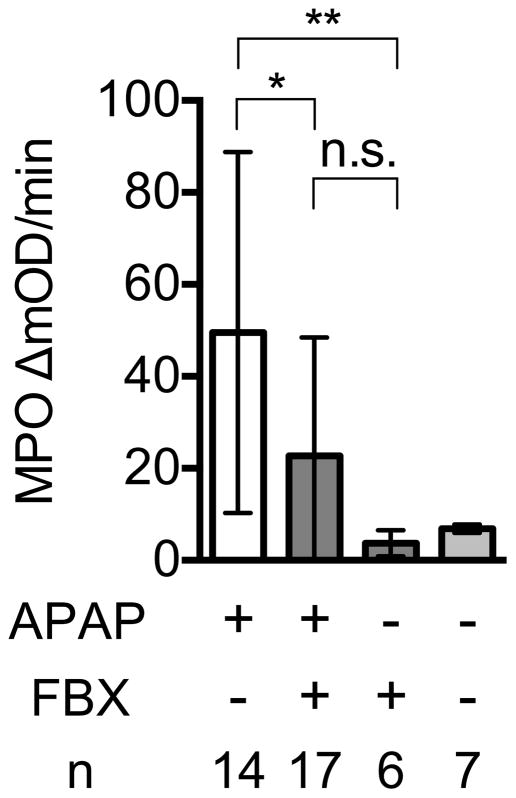
Myeloperoxidase activity in lung tissue 6 h after acid challenge in the presence or absence of FBX treatment
Right lungs were resected from Febuxostat (FBX) or control mice 6 h post intratracheal injection of acid or no acid injection. MPO activity in each lung homogenate was measured as the kinetic OD value change at 15-s intervals for 2 min 20 s. Means and S.D. are calculated from the accumulated data of 4 independent experiments. In individual experiments the number of mice per condition was 3–4 for experimental groups and 1 in the untreated controls. The bar shows the mean of the data and the limiting bars show the standard deviation. (n = total number of mice from the multiple experiments for each group). *P<0.05, **P<0.01 (Mann-Whitney U test)
3.3. Effect of Febuxostat on the neutrophilic inflammation to drug-induced liver injury
At high doses the drug acetaminophen causes hepatocyte necrosis (Chen et al., 2007; Jaeschke et al., 2012; Kono et al., 2010a). This is a consequence of the generation of a toxic metabolite, N-acetyl-p-benzo-quinone imine (NAPQI), when the drug is catabolized in the liver. Acetaminophen-induced hepatic necrosis stimulates inflammation and this process is caused in part by the release of uric acid from dead cells (Kono et al., 2010a). The end result of this process can by hepatic dysfunction or liver failure.
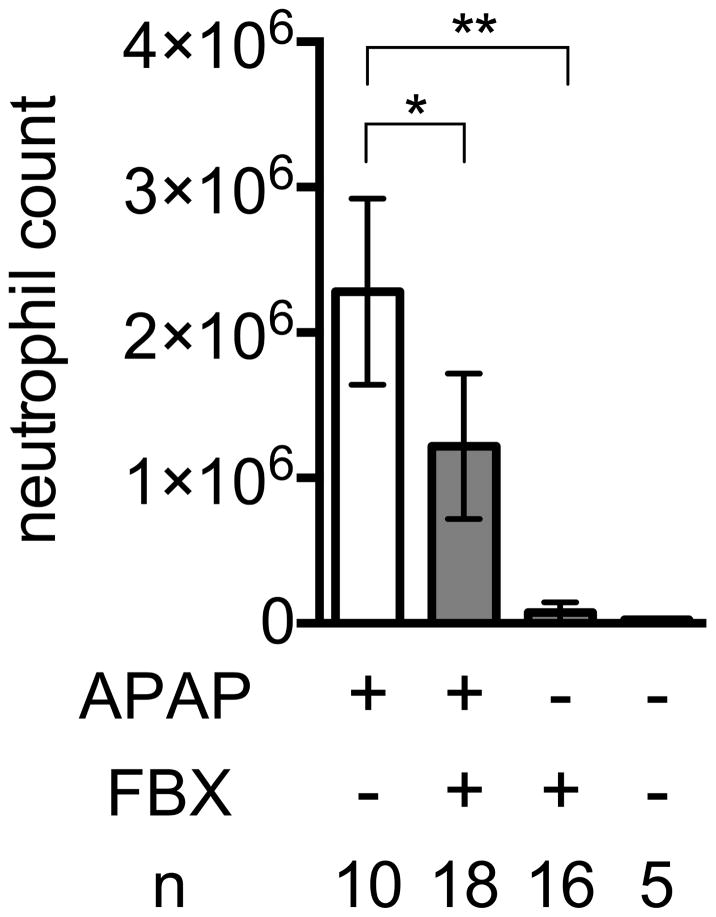
To determine whether Febuxostat could reduce the cell death-induced hepatitis from acetaminophen, control or Febuxostat-treated mice were treated with acetaminophen and the resulting neutrophilic inflammation quantified by flow cytometry and the measurement of myeloperoxidase. The livers of control acetaminophen-treated mice were infiltrated with neutrophils, as expected (Fig 4A). In contrast, the inflammatory response to hepatocyte injury was reduced in mice treated with Febuxostat (Fig 4A, B).
Fig. 4. Febuxostat inhibits acute liver inflammation induced by acetaminophen.
Fig. 4A
Neutrophil recruitment into damaged liver induced by acetaminophen in the presence or absence of febuxostat (FBX) treatment
C57BL/6 mice with or without pretreatment of febuxostat (FBX) were given 300mg/kg acetaminophen intraperitoneally and after 18 h their livers were perfused with HBSS buffer and digested with collagenase. The number of neutrophils and macrophages in nonparenchymal cells of their digested liver homogenates was quantified by flow cytometry. Means and S.D. were calculated from the accumulated data of 5 independent experiments. In individual experiments the number of mice per condition was 2–4 for experimental groups and 1 in the untreated controls. The bar shows the mean of the data and the limiting bars show the standard deviation. (n = total number of mice from the multiple experiments for each group). *,** P<0.05 (one-way ANOVA)
Fig. 4B
Effect of Febuxostat on myeloperoxidase (MPO) activity in acetaminophen-damaged livers.
18 h after acetaminophen administration, livers were perfused with HBSS buffer and homogenated in MPO buffer. After adding the substrate O-dianisidine and the developer H2O2, the kinetic change of OD450nm in liver homogenates (200 mg/ml) for 2 min 20 sec was analyzed on a plate reader. Means and S.D. were calculated from the accumulated data of 5 independent experiments. In individual experiments the number of mice per condition was 2–4 for experimental groups and 1–2 in the untreated controls. The bar shows the mean of the data and the limiting bars show the standard deviation. (n = total number of mice from the multiple experiments for each group). *,** P<0.05 (one-way ANOVA)
3.4. Febuxostat does not inhibit inflammation induced by zymosan
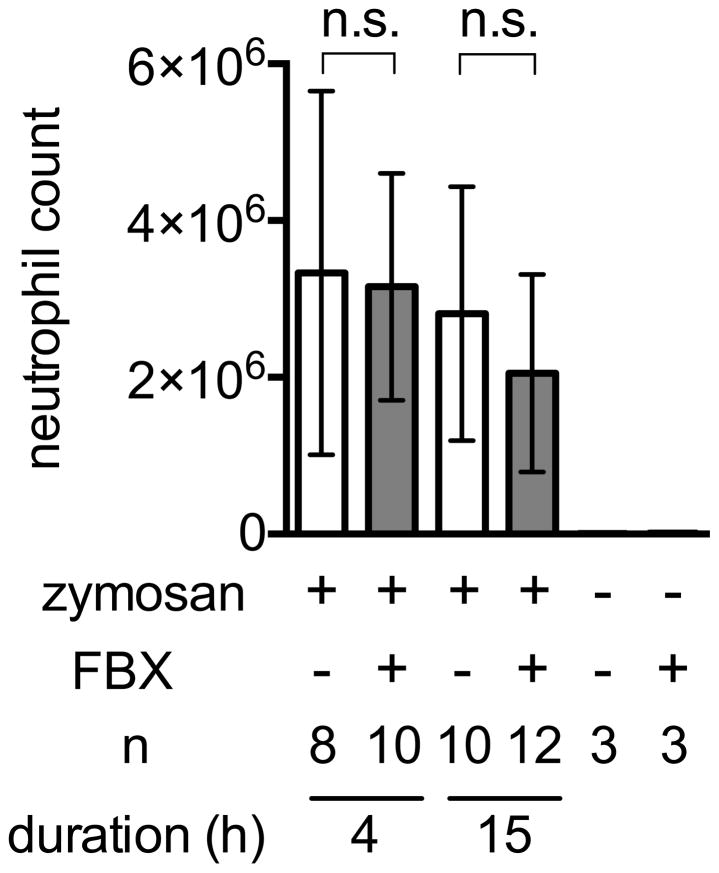
The results in the acid-induced lung and acetaminophen-induced liver injury models indicated that Febuxostat is reducing inflammation. This is consistent with this drug’s effect on lowering the intracellular levels of the pro-inflammatory DAMP uric acid. To investigate whether this agent might also have more general anti-inflammatory effects, we investigated whether it would reduce inflammation to a microbial stimulus (Cash et al., 2009). For this purpose, we injected zymosan (yeast cell wall) into the peritoneum of Febuxostat-treated and control mice. The ensuing inflammation was not significantly reduced in the Febuxostat treated animals (Fig. 5) although Febuxostat lowered their serum uric acid levels (data not shown). These results indicate that Febuxostat is not a general anti-inflammatory agent.
Fig. 5. Effect of Febuxostat on zymosan-induced peritonitis.
Peritoneal lavage was harvested from the mice given 100μg zymosan at indicated timing and the number of infiltrated neutrophils was quantified by flow cytometry. Means and S.D. were calculated from the accumulated data of 3 independent experiments. In individual experiments the number of mice per condition was 2–4 for experimental groups and 1 in the untreated controls. The bar shows the mean of the data and the limiting bars show the standard deviation. (n = total number of mice from the multiple experiments for each group).
4. Discussion
Necrotic cell death induces inflammation and the inflammation can cause symptoms and further damage tissue (Rock et al., 2011). Tissue infiltrating neutrophils are among the most damaging components of the acute inflammatory response. Therefore, agents that block cell death-induced neutrophilic inflammation might be useful therapeutically. The inflammation caused by cell death is caused in part by the release of intracellular stores of uric acid and potentially also by the continued catabolism of purines by xanthine oxidase in dead cells (Kono et al., 2010a). The xanthine oxidase inhibitor Febuxostat is used to reduce uric acid levels in humans and is a generally well tolerated drug, with no-to-limited side effects (Becker et al., 2005). Therefore, in this report we evaluated the potential of Febuxostat to lower intracellular pools of uric acid and to inhibit inflammation.
We found that Febuxostat was not a general anti-inflammatory agent, as it did not significantly reduce the inflammation stimulated by zymosan (Fig. 5). However, it significantly reduced the acute neutrophilic inflammatory response to lung injury by acid (Fig. 3A, B). It has previously been shown that reducing uric acid levels in vivo with uricase or allopurinol resulted in reduced pulmonary inflammation in response to the lung-damaging chemotherapeutic agent Bleomycin (Gasse et al., 2009). Thus our results extend earlier findings to another form of pulmonary injury that is clinically a much more common event than Bleomycin injury (Matute-Bello et al., 2008). It also shows the efficacy of a different uric acid lowering regimen with an orally bioavailable drug that is already used in humans for other indications. Overall these findings indicate that uric acid is an important proinflammatory DAMP in the lung and liver.
Our findings with Febuxostat in the lung injury model are consistent with those observed in the acetaminophen liver injury experiments (Fig. 4A, B). We found that Febuxostat reduce inflammation to the drug induced tissue necrosis. This is also consistent with earlier studies that had shown that lowering uric acid by other methods reduced inflammation in this same model. The effect in the present study correlated with the finding that Febuxostat was as effective at lowering tissue levels of uric acid in the lung as in the liver. In other words it appears uric acid levels are reduced enough to limit the uric acid-induced inflammation in both tissues.
Orally administrated Febuxostat distributes systemically and reduces ex vivo secretion of uric acid by adipose tissue of obese ob/ob mice and ameliorates damage in the lung and liver that is associated with ischemic intestinal injury, supporting our idea (Shafik, 2013; Tsushima et al., 2013). Our findings point to the potential usefulness of this drug for reducing of cell death-induced inflammation.
Supplementary Material
Key Messages.
Uric acid is an important mediator in acid-induced acute lung injury and acetaminophen liver damage.
Febuxostat lowers uric acid level in damaged tissues, leading to reduction of acute neutrophilic response.
Febuxostat may inhibit extra-articular inflammation that occurs in gout.
List of chemical compounds.
febuxostat: CID 134018
allopurinol: CID 2094
acetaminophen: CID 1983
Acknowledgments
Funding Statement.
This work was supported by funding from Takeda Pharmaceuticals U.S.A., Inc. and grant 5R01AI078287-05 to KLR from the NIH.
Footnotes
Conflict of Interest
All the authors declared no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Becker MA, Schumacher HR, Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, Streit J, Joseph-Ridge N. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353:2450–2461. doi: 10.1056/NEJMoa050373. [DOI] [PubMed] [Google Scholar]
- Cash JL, White GE, Greaves DR. Chapter 17. Zymosan-induced peritonitis as a simple experimental system for the study of inflammation. Methods in enzymology. 2009;461:379–396. doi: 10.1016/S0076-6879(09)05417-2. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty M, Jansen TL, Nuki G, Pascual E, Perez-Ruiz F, Punzi L, So AK, Bardin T. Gout: why is this curable disease so seldom cured? Annals of the rheumatic diseases. 2012;71:1765–1770. doi: 10.1136/annrheumdis-2012-201687. [DOI] [PubMed] [Google Scholar]
- Gasse P, Riteau N, Charron S, Girre S, Fick L, Petrilli V, Tschopp J, Lagente V, Quesniaux VF, Ryffel B, Couillin I. Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. Am J Respir Crit Care Med. 2009;179:903–913. doi: 10.1164/rccm.200808-1274OC. [DOI] [PubMed] [Google Scholar]
- Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011;364:1844–1854. doi: 10.1056/NEJMra0904569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Williams CD, Ramachandran A, Bajt ML. Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity. Liver international: official journal of the International Association for the Study of the Liver. 2012;32:8–20. doi: 10.1111/j.1478-3231.2011.02501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono H, Chen CJ, Ontiveros F, Rock KL. Uric acid promotes an acute inflammatory response to sterile cell death in mice. The Journal of clinical investigation. 2010a;120:1939–1949. doi: 10.1172/JCI40124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono H, Karmarkar D, Iwakura Y, Rock KL. Identification of the cellular sensor that stimulates the inflammatory response to sterile cell death. Journal of immunology. 2010b;184:4470–4478. doi: 10.4049/jimmunol.0902485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurts C, Panzer U, Anders HJ, Rees AJ. The immune system and kidney disease: basic concepts and clinical implications. Nat Rev Immunol. 2013;13:738–753. doi: 10.1038/nri3523. [DOI] [PubMed] [Google Scholar]
- Majno G, Joris I. Cells, tissues, and disease: principles of general pathology. 2. Oxford University Press; New York: 2004. [Google Scholar]
- Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. American journal of physiology Lung cellular and molecular physiology. 2008;295:L379–399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neogi T. Clinical practice. Gout N Engl J Med. 2011;364:443–452. doi: 10.1056/NEJMcp1001124. [DOI] [PubMed] [Google Scholar]
- Richette P, Bardin T. Gout. Lancet. 2010;375:318–328. doi: 10.1016/S0140-6736(09)60883-7. [DOI] [PubMed] [Google Scholar]
- Rock KL, Kataoka H, Lai JJ. Uric acid as a danger signal in gout and its comorbidities. Nature reviews Rheumatology. 2013;9:13–23. doi: 10.1038/nrrheum.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock KL, Lai JJ, Kono H. Innate and adaptive immune responses to cell death. Immunological reviews. 2011;243:191–205. doi: 10.1111/j.1600-065X.2011.01040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock KL, Latz E, Ontiveros F, Kono H. The sterile inflammatory response. Annu Rev Immunol. 2010;28:321–342. doi: 10.1146/annurev-immunol-030409-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafik AN. Febuxostat improves the local and remote organ changes induced by intestinal ischemia/reperfusion in rats. Digestive diseases and sciences. 2013;58:650–659. doi: 10.1007/s10620-012-2391-1. [DOI] [PubMed] [Google Scholar]
- Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- Tsushima Y, Nishizawa H, Tochino Y, Nakatsuji H, Sekimoto R, Nagao H, Shirakura T, Kato K, Imaizumi K, Takahashi H, Tamura M, Maeda N, Funahashi T, Shimomura I. Uric acid secretion from adipose tissue and its increase in obesity. The Journal of biological chemistry. 2013;288:27138–27149. doi: 10.1074/jbc.M113.485094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.