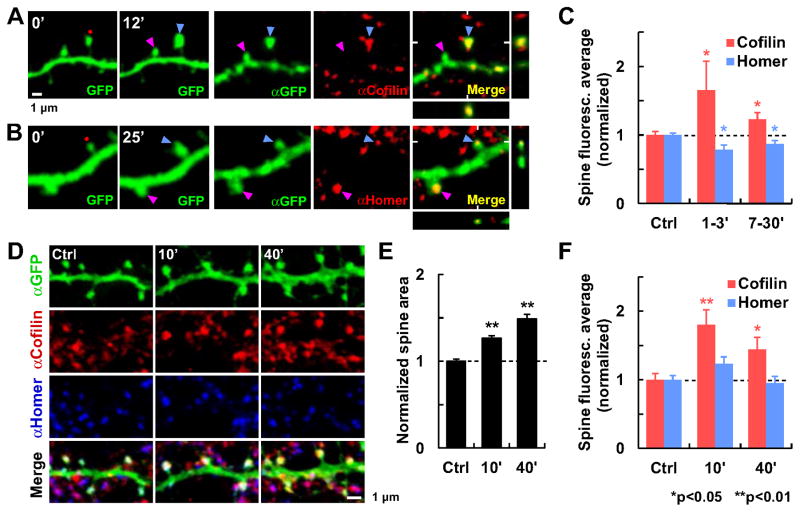
Fig. 3. Redistribution of endogenous cofilin-1 and Homer1b during sLTP.
Subcellular localization of endogenous cofilin-1 and Homer1b were detected by immunohistochemistry after two types of sLTP induction.
(A–C) sLTP was induced in single spines by glutamate uncaging (red dot) in organotypic hippocampal slices. (A–B) Examples of stimulated (blue arrowhead) and unstimulated (pink arrowhead) spines monitored by time-lapse live 2P imaging of GFP up to 12 min (A) or 25 min (B)after sLTP induction. Slices were subsequently fixed and immunostained for GFP (αGFP) and (A) cofilin-1 (αCofilin) or (B)Homer1 (αHomer). XZ and YZ projections are also shown. (C) Quantification of the spine protein concentration measured as the average immunofluorescence (total intensity in the spine head divided by spine area; mean ± SEM) of potentiated spines at two time periods (1–3 min [cofilin, n=8; Homer, n=7] or 7–30 min after induction [cofilin, n=32; Homer, n=25]) normalized to unstimulated spines (Ctrl; cofilin, n=85; Homer, n=118) from the same optical section.
(D–F) Chemical sLTP was induced by application of glycine to dissociated hippocampal cell cultures. (D) Examples of cultures fixed and immunostained for GFP, cofilin-1 and Homer1, before (Ctrl) or at different time points (10 or 40 min) after sLTP induction. (E) Quantification of the increase in spine area normalized to unstimulated spines (n=38 cells). (F) Quantification of the averaged immunofluorescence in the spine head in potentiated cultures at 10 min (n=23 cells) or 40 min (n=20) after stimulation, normalized to unstimulated cultures (Ctrl; n=22). * Significant difference with respect to Ctrl. See also Fig. S3.