Abstract
Liver disease is one of the major causes of morbidity and mortality across the world. According to WHO estimates, about 500 million people are living with chronic hepatitis infections resulting in the death of over one million people annually. Medicinal plants serve as a vital source of potentially useful new compounds for the development of effective therapy to combat liver problems. Moreover herbal products have the advantage of better affordability and acceptability, better compatibility with the human body, and minimal side effects and is easier to store. In this review attempt has been made to summarize the scientific data published on hepatoprotective plants used in Saudi Arabian traditional medicine. The information includes medicinal uses of the plants, distribution in Saudi Arabia, ethnopharmacological profile, possible mechanism of action, chemical constituents, and toxicity data. Comprehensive scientific studies on safety and efficacy of these plants can revitalise the treatment of liver diseases.
1. Introduction
Since the dawn of history plants have played an important role in the treatment of human ailments. By trial and error the ancient population was relieving their sufferings by using herbs in a very primitive way. The history of many drugs which are in practice today could be traced back to the Hellenic civilization; drugs like castor oil, opium, olive, anise, peppermint, saffron, henbane, acacia, and yeast are mentioned in Egyptian Ebers Papyrus (1500 B.C.). Babylonians and Assyrians have mentioned a large number of herbal medicines, for example, coriander, cinnamon, liquorice, and so forth. Thya, written by the Chinese physician Chou Kung, in about 1100 B.C., describes the use of a number of plant drugs. The books of Sustruta, written in India at the beginning of the Christian era, describe some seven hundred herbal medicines. According to the recent analysis, man is recycled plant and plants fulfill a variety of human needs, as they are the source of nourishment, health, and pleasure [1]. Even the lowest form of plant life can be vital, and penicillin is only one—no doubt—the most famous antibiotics.
1.1. Greeko-Arab System of Medicine
Greeko-Arab system of medicine is a well-documented system of traditional medicine which was originated by Greek physicians and philosophers and enriched by Arabs. It was the work of the Greek philosopher-physician Hippocrates (460–377 B.C.), who freed herbal supplements from the realm of superstition and magic and gave it the status of science. He considered illness to be natural rather than a supernatural phenomenon, and he strongly suggested that herbal supplements should be administered without ritual ceremonies or magic [1]. After Hippocrates, Galen (131–200 A.D.) stands out for his contribution to traditional medicine. Galen introduced and practiced herbal medicine in preIslamic Egypt, serving as royal court physician to the king of Egypt [2]. Under his patronage, hundreds of new herbal supplements were researched, experimented, and developed for the treatment of almost all types of diseases. Galen's contributions in herbal remedies is highly regarded, even today the term galenical is applied to simple vegetable extractives. Arabs like Rhazes (850–932 A.D.), Avicenna (980–1037 A.D.), Al-Bitar (1180–1248 A.D.), and Al-Antaki (1510–1587) constructed an imposing edifice of Arab traditional medicine. “Avicenna” (the western name for Abu Sina), an Arab philosopher and physicist who wrote “Kitab-al-shifa” (The Canon of Medicine), is highly noteworthy. According to Greeko-Arab system of medicine, disease is a natural process resulting from the imbalance of various humors in the body. The humoral theory presupposes the presence of four humors: dam (blood), balgham (phlegm), safra (yellow bile), and sauda (black bile) in the body. The temperaments of persons are expressed by the words sanguine, phlegmatic, choleric, and melancholic according to the preponderance of the following four humors in their body, namely, blood, phlegm, yellow bile, and black bile, respectively. The humors themselves are assigned temperaments. Blood is hot and moist, phlegm is cold and moist, and yellow bile is hot and dry. According to the Greeko-Arab system herbs may restore humor imbalance and cure the diseases [3].
Greeko-Arab physicians identified the liver as one of the three principal organs of the body, along with the heart and the brain. According to Galen the liver is the “master organ” of the human body, arguing that it emerges before all other organs in the fetus formation. In his book entitled “On the Usefulness of the Parts of the Body” Galen described the liver as warm and moist organ involved in blood formation and principle instrument of sanguification. According to Avicenna liver is “the seat of nutritive or vegetative faculties” and “the seat of manufacture of the dense part of the humors”. According to Arab physicians, malfunction of liver may lead to a variety of diseases which may be corrected by appropriate herbal intervention.
1.2. Liver Diseases and Their Global Burden
Liver is the largest and most vital organ of the human body. Besides its crucial role in the metabolism of nutrients, liver is responsible for biotransformation of drugs and chemicals thereby protecting body against toxic foreign materials. In this process the liver is exposed to high concentration of toxic chemicals and their metabolites which may cause liver injury. There are more than hundred well known liver diseases with diversified etiopathology. The most frequent causes of hepatic disease include infectious agents (especially hepatitis viral A, B, and C), obesity related fatty liver disease, xenobiotics (alcohol, drugs, and chemicals) induced liver injury, inherited and genetic defects related liver diseases, autoimmune hepatitis, liver cirrhosis, and primary or secondary liver cancer.
Liver diseases are one of the leading causes of morbidity and mortality across the world. Around 1.3 million deaths worldwide are due to chronic viral hepatitis. Many clinic-led researchers have found that liver related mortality is as high as fourth for some age group and eighth overall. According to WHO estimates, about 1.4 million cases of hepatitis A occur annually and 2 billion people worldwide are infected with the hepatitis B virus. About 350 million live with chronic infection and 600,000 persons die each year due to the acute or chronic consequences of hepatitis B. About 130–170 million people are chronically infected with hepatitis C virus, and more than 350,000 people die from hepatitis C-related liver diseases each year [4]. The recent statistics clearly show that global burden of liver disease has increased over time with a huge impact on overall world population.
1.3. A Tilt towards Herbal Drugs
The treatment options for common liver diseases are limited due to the lack of hepatoprotective drugs in allopathic medicine. Moreover therapies developed along the principle of western medicine are often limited in efficacy, carry the risk of adverse effects, and are often too costly, especially for the habitants of developing world. For example, the effectiveness of treatments such as those using corticosteroids and interferon is inconsistent, carried the risk of adverse events, and is often too costly [5]. On the other hand plant derived compounds are easily accessible and affordable. There is a deep belief that herbal remedies symbolize safety because they are “natural” and fit into the image of a gentle and, therefore, harmless alternative to synthetic drugs. No doubt that herbs are staging a comeback and herbal “renaissance” is happening all over the world. Several recent surveys from Europe and the United States have demonstrated a sharp rise in the popularity and use of botanical drugs within a few years, with up to 65% of liver patients taking herbal preparations. The fact is that reliable hepatoprotective drugs are explicitly inadequate, and the search for natural herbal drugs has intensified in the recent decades.
In this review we summarized the scientific data published on thirty-five hepatoprotective plants used in Saudi Arabian traditional medicine, description of the plants and their distribution in Saudi Arabia, medicinal uses, experimental pharmacological studies, possible mechanism of action, chemical constituents, and toxicity studies.
2. Methodology
A list of hepatoprotective plants used in Saudi Arabia was prepared based on a nationwide survey of herbal drug used in traditional medicine for liver ailment by
interviewing the patients visiting primary care centres of military hospitals of different regions of Saudi Arabia,
review of traditional medicinal books/publications and folklore information.
A thorough of survey of literature on the pharmacological profile of these plants was undertaken to collect the published data for the period between 1975 and 2014 AD by using “Pubmed” and “Google Scholar” search engines. Attempt was made to determine if these plants have been tested for hepatoprotective activity using well-established experimental models including carbon tetrachloride (CCl4), thioacetamide, paracetamol, ethanol, and morphine induced liver damage. The liver enzymes including aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (APT), total protein (TP), and albumin (Alb) were used as a marker of liver injury. Literature search also included reversal of toxin induced histopathological changes by plant drugs.
An attempt has been made to illustrate possible mechanism of hepatoprotective herbs with special reference to their antioxidant (ability to normalize oxidative stress markers) and inflammatory mediators. Available data about the chemical constituent of the hepatoprotective plants and their toxicity has also been presented.
Briefly, this review summarises the information about 35 hepatoprotective herbal drugs used in Saudi traditional medicine (Table 1) for the treatment of liver diseases including their botanical name, family, and part of the plant used, distribution of plants in Saudi Arabia, and their use in traditional medicine. The results of hepatoprotective studies on each plant, possible mechanism of action, and their chemical composition and toxicity data have been presented.
Table 1.
Saudi herbal drug with hepatoprotective activity.
| Plant name | Traditional uses | Chemical constituent | Reference |
|---|---|---|---|
| Apium graveolens | Liver and spleen disorders, jaundice, rheumatism, gout, and inflammatory diseases | Limonene, p-dimethyl styrene, n-pertyl benzene, caryophyllene, a-selinene, n-butyl phthalide, and sedanenolide | [7–9, 13] |
| Artemisia scoparia | Jaundice and liver disorders | Hyperin, eupafolin, pedalitin, 5,7,2′,4′-tetrahydroxy-6,5′-dimethoxyflavone, camphor, and 1,8-, and beta-caryophyllene | [16, 17, 26] |
| Bacopa monnieri | Jaundice, liver diseases, spleen disorders, and digestive problems | Brahmine, bacosides-a, nicotine, herpestine, d-mannitol, and hersaponin | [27, 28, 34] |
| Balanites aegyptiaca | Jaundice, liver disorders, and spleen problems | Quercetin 3-glucoside, quercetin-3-rutinoside; 3-glucoside, 3-rutinoside, 3-7-diglucoside, and 3-rhamnogalactoside | [35, 41] |
| Beta vulgaris | Spleen, liver problems, and inflammatory disorders | Betacyanins, betaxanthins, oxalic acid, and ascorbic acid | [42–44, 47] |
| Boerhavia diffusa | Jaundice and other liver diseases, internal inflammation, gall bladder problem, and spleen disorders | Punarnavine, boeravinones, flavonoids, amino acids, lignans, and tetracosanoic, esacosanoic, stearic, and ursolic acids | [48] |
| Camellia sinensis | Obesity/weight loss, arthritis, and other inflammatory conditions | Caffeine, theophylline, and theobromine | [54, 62] |
| Clitoria ternatea | Liver diseases | Taraxerol, taraxerone, ternatins, flavonoids, saponins, and tannins | [63, 64, 66, 69] |
| Commiphora opobalsamum | Stomach, jaundice, liver diseases, joint pain, and inflammatory disorders | Flavonoids, saponins, volatile oil, sterol, and/or triterpenes | [72, 73] |
| Curcuma longa | Loss of appetite, jaundice, liver problems, gall bladder disorders, and arthritis | Curcumin, demethoxycurcumin, and bis-demethoxycurcumin | [76–78] |
| Eruca sativa | General tonic, liver, and intestinal disorders | Glucosinolates, quercetin, and erucic acid | [82–84, 86] |
| Ficus carica | Liver disease, stomach ailments, digestive problems, obesity, and inflammatory diseases | Psoralen, mucilages, flavonoids, vitamins, nicotinic acid, tyrosine, ficusin, bergaptene, stigmasterol, taraxasterol, beta-sitosterol, rutin, and sapogenin | [89–93, 97] |
| Grewia mollis | Liver disease, abdominal problems, arthritis, and inflammatory conditions | Luteolin, tetrahydroxyflavone, 7β-hydroxy-23-enedeoxojessic acid, 7β-hydroxy-23-deoxojessic acid, β-sitosterol, and β-sitosterol-3-o-glucoside | [99–102] |
| Grewia tenax | Liver disorders, jaundice, and inflammatory condition | Betulin, triacontan-1-ol, α-amyrin, β-amyrin, β-sitosterol, lupenne, erythrodiol, and tetratriacont-21-ol-12-one | [4, 104, 105] |
| Haloxylon salicornicum | Jaundice, gall bladder stones, liver diseases, digestive disorders, inflammatory disorder, and joint diseases | Ursolic acid, 7-hydroxy-4-triacontanone, 24-hydroxy-4-octacosanone, 1-triacontanol, β-amyrin, 24-ethylcholesta-3,5-diene, 24-nor-12-ursene, β-sitosterol, and β-sitosterol | [106–111] |
| Hypericum perforatum | Jaundice, liver diseases, gall bladder stones, rheumatoid arthritis, and inflammatory conditions | Rutin, hypericin, pseudohypericin, hyperforin, adhyperforin, quercetin, hyperoside, campferol, myricetin, amentoflavone, i3, kielcorin, and norathyriol | [112–115] |
| Juniperus procera | Liver disease, jaundice, digestive problems, inflammatory diseases, and ulcers | β-peltatin a, methyl ether and deoxypodophyllotoxin, and totarol | [115–117] |
| Lepidium sativum | Jaundice, liver problems, spleen diseases, gastrointestinal disorders, arthritis, and inflammatory disorders | Alkaloids, saponins, anthracene glycosides, carbohydrates, proteins, amino acids, flavonoids, and sterols | [53, 118] |
|
Moringa oleifera
(seed oil) |
Liver disease, lipid disorders, arthritis, and inflammatory disorders | β-carotene, protein, and vitamin c | [119–122] |
| Nigella sativa | Liver tonics, digestive, anti-inflammatory, immunostimulant, and remedy for jaundice | Thymoquinone, thymohydro quinine, dithymoquinone, p-cymene, carvacrol, and 4-terpineol | [123–125] |
| Peganum harmala | Jaundice, digestive disorders, liver disease, and arthritis | Harmaline, harmine, harmalol, and tetrahydroharmine | [126–131] |
| Pergularia daemia | Jaundice, liver diseases and inflammatory disorders | Cardenolides, alkaloid, saponins, triterpenes, and steroidal compounds | [132–135] |
| Petroselinum crispum | Liver diseases, constipation, flatulence, jaundice, colic pain and rheumatism | Flavone glycosides | [136–140] |
| Phyllanthus maderaspatensis | Emetic and purgative, constipation, digestion and abdominal pain liver disorders, rheumatism and inflammatory diseases | Essential oil, mandarin, mucilage, and β-sitosterol | [141–144] |
| Pimpinella anisum | Digestive, carminative, antispasmodic and for liver disorders | Trans-anethole and palmitic and oleic acids | [145–147] |
| Portulaca oleracea | Liver disorders, gastrointestinal problems and inflammatory disorders | Omega-3 fatty acids, alpha-linolenic acid, and vitamins a, b, and c | [82, 148–151] |
| Rhazya stricta | Stomach problems, liver diseases and inflammatory disorders | Akuammidine, bhimberine, rhazimol | [36, 152–155] |
| Smilax regelii | Liver diseases, arthritis and inflammatory conditions | Saponins flavonoids, tannins, sterols, and triterpenes | [156–160] |
| Solanum nigrum | Liver disorders, jaundice and cirrhosis, inflammatory disorders, rheumatism and swellen joints | Glycoalkaloids, glycoproteins, polysaccharides, gallic acid, catechin, protocatechuic acid, caffeic acid, epicatechin, rutin, and naringenin. | [161–165] |
| Suaeda maritima | Liver, heart and lipid disorders | Alkaloid, flavonoid, and tannins | [166–168] |
| Tamarix nilotica | Liver, stomach and inflammatory problems | Kaempferol, syringaresinol, isoferulic acid, niloticol, 3-hydroxy-4-methoxycinnamaldehyde, methyl and ethyl esters of gallic acid, para-methoxygallic acid, quercetin 3-oglucuronides, 3-o-sulphated kaempferol, and 7,4′-dimethyl ether | [169–171] |
| Tephrosia purpurea | Jaundice, liver, biliary and splenic disease, and inflammatory disorders | β-sitosterol, quercetin, lupeol, rutin, delphinidin chloride, cyanidin chloride, isolonchocarpin, lanceolatins a and b, pongamol, karangin, kangone, 5,7-dimethoxy-8-flavanone, 2-methoxy-3,9-dihydroxycoumestone | [172–176] |
| Teucrium polium | Liver diseases, inflammatory disorders, stomach and intestinal troubles and rheumatism | Caryophyllene, cedrol, a-epi-cadinol, and e-g-bisabolol | [177–181] |
| Trianthema Portulacastrum | Liver diseases and pain | Flavonoid, steroids, fats, terpenes, carbohydrates, tannins, and alkaloids | [182, 183] |
| Tribulus terrestris | Tonic, diuretic, and aphrodisiac | Tigogenin, neotigogenin, terrestrosid F, and gitonin | [184, 185] |
3. Results
3.1. Apium graveolens Linn
Apium graveolens Linn. (family: Apiaceae) locally known as “Karfas” is a biennial or perennial glabrous herb with a heavy aromatic smell found in Najd region of Saudi Arabia [6]. Seeds of A. graveolens have been widely used in traditional medicine for the treatment of liver and spleen disorders, jaundice [7], rheumatism, gout, and other inflammatory diseases [8, 9].
The hepatoprotective activity of the methanolic extract of A. graveolens seed has been studied against CCl4 [10, 11] and paracetamol [11, 12] induced liver damage. A. graveolens extract dose dependently attenuated the toxin induced biochemical (serum AST, ALT, APT, TP, and albumin) and histopathological changes in liver tissues. The protective activity of A. graveolens was comparable with silymarin a well-established hepatoprotective herbal drug [10, 12]. Acute toxicity studies on A. graveolens extract in rats showed no adverse symptoms. Lethal dose in 50% of rats (LD50) was found to be of 7.5 g/kg body weight (b.w.) clearly suggesting its large margin of safety [12]. Chronic toxicity studies on the extract also revealed no delirious effect or mortality over a period of 14 days [13].
The hepatoprotective effect of A. graveolens Linn. may be attributed to its anti-inflammatory [14] and antioxidant activities [15]. Phytochemical screening showed the presence of flavonoids, tannins, volatile oils, alkaloids, sterols, and triterpenes. Detailed chemical studies also showed the presence of limonene, p-dimethyl styrene, N-pertyl benzene, caryophyllene, α-selinene, N-butyl phthalide, and sedanenolide [13].
3.2. Artemisia scoparia Waldst.et Kit
Artemisia scoparia Waldst.et Kit. (family: Compositae) locally known as “Baeiteran/A'weejan” is an annual herb mostly found in eastern and Najd region of Saudi Arabia [6]. The aerial part of A. scoparia has long been used in folk medicine for the treatment of jaundice and other liver disorders [16, 17].
The hepatoprotective activity of hydroalcoholic extract of aerial parts A. scoparia was investigated against CCl4 [18, 19] and paracetamol [20] induced liver damage. The extract dose dependently attenuated hepatotoxin induced biochemical parameters (rise in serum AST and ALT) and prolongation of phenobarbital induced sleeping time clearly indicating its hepatoprotective action. Hepatotoxins like CCl4 and paracetamol significantly reduced the activity of drug metabolizing enzymes in liver, leading to the slowing of drug metabolism resulting in increased level of drugs such as barbiturates which results in prolongation of their pharmacological activity (sleeping time). Reversal of barbiturate induced sleeping time suggests hepatoprotective effect of A. scoparia [18]. A. scoparia also has a potent choleretic activity as evident from significant increase in bile volume, bile acid, and bile salt [21]. Recent pharmacological studies also showed anti-inflammatory [22] and antioxidant [23] activities of A. scoparia which may contribute to its hepatoprotective activity. Although the plant is recognised as antihelmintic, its mammalian toxicity is negligible [24]. Some cases of dermatitis and allergic reaction have been reported [25]. Phytochemical studies on aerial part of A. scoparia showed the presence of hyperin, eupafolin, pedalitin, 5,7,2′,4′-tetrahydroxy-6,5′-dimethoxyflavone, camphor, 1,8-, beta-caryophyllene, cirsilineol, cirsimaritin, arcapillin, and cirsiliol [26].
3.3. Bacopa monnieri Linn
Bacopa monnieri Linn. (family: Plantaginaceae) locally known as “Farfakh” is a small creeping glabrous perennial herb. In Saudi Arabia the plant grows in Tabuk, Al Jauf, Sakakah, northern Hejaz, and eastern region [6]. B. monnieri is largely treasured as a revitalizing herb. In traditional medicine it has been used for more than 3000 years for the treatment of jaundice, liver diseases, spleen disorders, and digestive problems [27, 28].
Hepatoprotective activity of ethanolic extract of whole plant of B. monnieri has been studied against nitrobenzene [29] and morphine [30] induced liver toxicity. The extract significantly attenuated hepatotoxin induced changes in biochemical parameters (sera AST, ALT, and APT) and histopathological changes in liver tissues. Ethanolic extract of B. monnieri also showed significant antioxidant [30] and anti-inflammatory [31] activities, which may contribute to its hepatoprotective activity. Acute toxicity studies showed no deterious effect in pharmacological doses. The single dose LD50 was found to be 2400 mg/kg b.w. in rats. In a chronic toxicity study in rats, B. monnieri was found to be well tolerated up to the dose of 500 mg/kg b.w. for 3 months [32]. Phytochemical analysis on plant of B. monnieri showed the presence of alkaloid (brahmine), bacosides, nicotine, herpestine, D-mannitol, hersaponin, stigmosterol, beta-sitosterol, and bacosaponins [30, 33]. Bacoside, a major constituent of brahmi, has been shown to possess significant anticancer activity against liver tumors in rats [34].
3.4. Balanites aegyptiaca Linn
Balanites aegyptiaca Linn. (family: Zygophyllaceae) locally known as “Sidrul Kajjab” is a small shrub with thorn on stem. In Saudi Arabia, it is abundant in southern part of Hejaz ranging from Jeddah to Yemen border [6]. The bark, unripe fruits, and leaves of the B. aegyptiaca are used in folk medicine for the treatment of jaundice, liver disorders, and spleen problems [35].
The effect of ethanolic extracts of bark of B. aegyptiaca has been investigated against paracetamol [36] and CCl4 [37] induced hepatotoxicity in rats. The extract dose dependently attenuated the hepatotoxin induced biochemical (serum AST, ALT, ALP, and bilirubin) and histopathological changes in liver which was comparable with silymarin. The extract also reversed toxin induced prolongation of pentobarbital sleeping time in rats. The purified fractions of B. aegyptiaca possess significant antioxidant [38] and anti-inflammatory [39] activities which may contribute to its hepatoprotective action. Administration of 5% seed oil in diet produced mild toxicity in rats [40]. Phytochemical studies on B. aegyptiaca showed the presence of flavonoids, saponins, quercetin 3-glucoside, quercetin-3-rutinoside, 3-glucoside, 3-rutinoside, 3-7-diglucoside, and 3-rhamnoglucoside [41].
3.5. Beta vulgaris Linn
Beta vulgaris Linn. (family: Amaranthaceae) locally known as “shahya” is an annual or biennial herb found mostly in North Hejaz and Eastern Najd region of Saudi Arabia [6]. Beta vulgaris is extensively cultivated as an article of food and the roots are used for the production of sugar. The plant root has been used in traditional medicine for a wide range of diseases including spleen and liver problems and inflammatory disorders [42–44].
Oral administration of the ethanolic extract of Beta vulgaris roots exhibited significant and dose dependent hepatoprotective activity against CCl4 induced liver damage in rats [45]. The hepatoprotective activity of Beta vulgaris may be attributed to its antioxidant [46] and anti-inflammatory [14] activities. The plant is safe to use even in large doses. Phytochemical studies on roots of Beta vulgaris Linn. have shown the presence of betaine, betacyanins, betaxanthins, oxalic acid, and ascorbic acid [47].
3.6. Boerhavia diffusa Linn
Boerhavia diffusa Linn. (family: Nyctaginaceae) locally known as “maddad” is a tall glabrous plant with a forked herbaceous stem widely distributed in Abha, Bisha, Najran, and Hejaz region of Saudi Arabia [6]. Boerhavia diffusa has been widely used in traditional system of medicine for the treatment of jaundice and other liver diseases, internal inflammation, gall bladder problem, and spleen disorders [48].
Aqueous and ethanolic extracts of B. diffusa significantly attenuated acetaminophen [49] and ethanol [50] induced biochemical (rise of serum AST, ALT, APT, and bilirubin) and histopathological changes in liver suggesting its hepatoprotective action. The extract has been shown to possess significant antioxidant [49] and anti-inflammatory [51] activities which may contribute to its hepatoprotective activity. The oral LD50 for B. diffusa leaves in mice and rats was found to be 2000 mg/kg b.w. [52]. The aerial part of B. diffusa is a rich source of flavonoids, steroids, and alkaloids. Detailed phytochemical analysis showed the presence of campesterol, daucosterol, sitosterols, punarnavine, boeravinones A-F, borhavone, amino acids, lignans, and tetracosanoic, esacosanoic, stearic, and ursolic acids [48].
3.7. Camellia sinensis Linn
Camellia sinensis Linn. (family: Theaceae) locally known as “Shai.” The leaves and buds of this plant are used to produce the popular tea beverage. Our survey showed that Camellia sinensis is the second most commonly used herb by Saudi population for liver problems [53]. The decoction is used for obesity/weight loss, arthritis, and other inflammatory conditions and as anticancer [54].
The hepatoprotective activity of the aqueous extract of C. sinensis has been studied against experimentally induced liver damage in rats. The extract significantly attenuated CC14 induced biochemical (serum ALT, AST, ALP, total protein, and albumin) and histopathological changes in liver [55]. Tea decoction has been shown to possess significant antioxidant, anti-inflammatory, and immunomodulatory activities [56, 57], which may contribute to its hepatoprotective activity. The antioxidant and anti-inflammatory activity of tea has been attributed to saponin contents of C. sinensis [58]. High doses of tea may cause convulsion/stimulation of central nervous system (CNS) due to its caffeine contents [59]. Some cases of green tea induced liver toxicity have been reported [60, 61]. Phytochemical studies on aerial part of C. sinensis have shown the presence of saponins, flavonoids, quercetine, quercitrin, rutin, catechin, caffeine, theophylline, and theobromine [62].
3.8. Clitoria ternatea Linn
Clitoria ternatea Linn. (family: Fabaceae) locally known as “Al-clitoria” is a perennial plant with big 5–7 elliptic to lanceolate leaflets abundant in southern Hejaz region of Saudi Arabia [6]. The leaves, seeds, and flowers are used in traditional medicine for liver diseases [63, 64].
Methanolic extract of C. ternatea (200 mg/kg) significantly attenuated CCl4 [65] and paracetamol [66] induced biochemical (serum ALT, AST, and bilirubin levels) and histopathological alterations in liver. “Ayush-Liv.04” a polyherbal formulation consisting of 20% C. ternatea leaves as one of its constituents also showed significant hepatoprotective activity against ethanol and CCl4 induced liver damage in rats [67]. C. ternatea possess significant anti-inflammatory [68] and antioxidant [69, 70] activities which may contribute to its hepatoprotective effects. Roots of C. ternatea did not show any toxicological signs or deaths up to doses of 3000 mg/kg b.w. [71]. Phytochemical studies on leaves of C. ternatea showed the presence of flavonoids, saponins, tannins, glycosides, quercetin, steroids, taraxerol, taraxerone, ternatins, and taraxerol [66, 69].
3.9. Commiphora opobalsamum Linn
Commiphora opobalsamum Linn. (family: Burseraceae) locally known as Ood-e-Balsan, Behsan, or Balessan is medicinal plant with small, thorny tree which grows widely in Mecca region of Saudi Arabia. Local folk healer uses it for the treatment of stomach, jaundice and liver diseases, joint pain, and inflammatory disorders [72, 73].
The hepatoprotective activity of ethanolic extract of C. opobalsamum was studied using an experimental model of hepatotoxicity in rats [72]. The extract dose dependently protected liver against CCl4 induced biochemical (serum AST, ALT, and APT) and prolongation of the barbiturate sleeping time. The extract also showed significant antioxidant [72] and anti-inflammatory [74] activities which may contribute to its hepatoprotective effects. Even the large doses of ethanolic extract of C. opobalsamum did not show adverse effects in rats [75]. Phytochemical studies on aerial part of C. opobalsamum showed the presence of saponins, volatile oil, sterol and/or triterpenes, friedelin, flavonoids, mearnsetin, and quercetin [72].
3.10. Curcuma longa Linn
Curcuma longa Linn. (family: Zingiberaceae) locally known as “curcum” is a small rhizomatous perennial herb [6]. The genus named Curcuma is the latinized form of the Arabic Al-Kurkum. For over 4000 years, it has been widely used in Asian traditional medicine for loss of appetite, jaundice, liver problems, gall bladder disorders, and arthritis [76, 77]. Experimental studies have substantiated its use as hepatoprotective and hypolipidemic [78].
Hepatoprotective effect of turmeric has been attributed to its antioxidant [79] and anti-inflammatory [80] properties. Sodium curcuminate, a salt of curcumin, also exerts choleretic effects by increasing biliary excretion of bile salts, cholesterol, and bilirubin, supporting its use for the treatment of cholelithiasis. Toxicity studies on C. longa in animals showed no adverse effect up to 2.5 g/kg b.w. [81]. In humans, large doses may cause gastric irritation. The healing effect of C. longa is attributed to polyphenolic curcuminoids including curcumin I, curcumin II, and curcumin III [78].
3.11. Eruca sativa Mill
Eruca sativa Mill. (family: Cruciferae) locally known as “Jarjeer” is a hairy plant having oblong leaves grows in northern Hejaz, Najd, and eastern region of Saudi Arabia [6]. In Greeko-Arab medicine, E. sativa is considered as general tonic [82]. It has been used for treatment of liver and intestinal disorders [83]. E. sativa has gained greater importance as a salad vegetable and spice, especially among Middle Eastern populations and Europeans. The leaves and seeds have been investigated for their hepatoprotective, anti-inflammatory, and antioxidant activities [84].
The ethanolic extract of E. sativa leaves and seeds showed significant hepatoprotective activity against CCl4 [84] and ethanol [85] induced liver injury. The E. Sativa extract also showed significant cytoprotective effect against liver cancer cells [86]. The hepatoprotective activity of E. Sativa may be attributed to its antioxidant [87] and anti-inflammatory [88] activities. It is an edible plant with no reported toxicity. Phytochemical studies on leaves of E. sativa have shown the presence of large amount of polyphenols, flavonoids, erucin, erysolin, glucosinolates, quercetins, erucic acid, and phenylethyl isothiocyanate [84, 86].
3.12. Ficus carica Linn
Ficus carica Linn. (family: Moraceae) locally known as “Hammat teen” is a shrub with milky big palmately lobed leaves found mostly in southern Hejaz and Najd region of Saudi Arabia [6]. The fig is cultivated as an edible fruit. The plant has been widely used in Greeko-Arab traditional medicine for the treatment of liver diseases, stomach ailments, digestive problems, obesity, and inflammatory disorders [89–92].
The hepatoprotective activity of various extracts of F. carica leaves and fruits have been experimentally confirmed against CCl4 [93, 94] and rifampicin [95] induced hepatotoxicity. The hepatoprotective activity of F. carica may be attributed to its marked anti-inflammatory [96] and antioxidant [97] activities. F. carica being an edible fruit is generally considered safe; however the unripe fruit may cause toxic effect and its sap may cause contact dermatitis [98]. Phytochemical studies on leaves and fruits of F. carica have shown the presence of flavonoids, vitamins, nicotinic acid, tyrosine, ficusin, bergaptene, stigmasterol, furocoumarin, psoralen, taraxasterol, beta-sitosterol, rutin, and sapogenin [93, 97].
3.13. Grewia mollis Juss
Grewia mollis Juss. (family: Malvaceae) locally known as “Nab'aaa” is a shrub/tree found mostly in Hejaz region of Saudi Arabia [6]. The leaves and bark of G. mollis have been used in traditional medicine for the treatment of liver diseases, abdominal problems, arthritis, and inflammatory conditions [99–101].
Methanolic extract of G. mollis leaves showed significant hepatoprotective activity against CCl4 induced liver injury [102]. G. Mollis extract possesses significant antioxidant [102] and anti-inflammatory [99] activities which may contribute to its hepatoprotective effects. The pharmacological effect of G. mollis may be attributed to its steroidal and/or triterpenoidal constituent which have proven to be anti-inflammatory activity [99]. High doses of G. mollis stem bark may cause mild adverse effects including impairment of liver function [103]. Phytochemical studies on leaves of G. mollis has shown the presence of luteolin, tetrahydroxyflavone, 7β-hydroxy-23-enedeoxojessic acid, 7β-hydroxy-23-deoxojessic acid, β-sitosterol, and β-sitosterol-3-O-glucoside [99, 102].
3.14. Grewia tenax Forsk
Grewia tenax Forsk. (family: Malvaceae) locally known as “khaddar/shohat” is a glabrous shrub found in southern Hejaz region of Saudi Arabia [6]. In traditional medicine leaves, root, and fruits of G. tenax are used for the treatment of digestive diseases, liver disorders, jaundice, and inflammatory conditions [4, 104].
The administration of ethanol extract of G. tenax significantly restored CCl4 induced biochemical (serum AST, ALT, APT, TB, and gamma-glutamyl transferase) and histopathological changes in rats. Reversal of pentobarbital-induced prolongation of narcolepsy by the extract also suggested its hepatoprotective effect. The chronic administration of extract significantly reduced cholesterol, low-density lipoproteins, and triglycerides level [105]. The hepatoprotective effect of G. tenax is attributed to antioxidant [105] and anti-inflammatory [105, 186] properties. Experimental studies in mice showed no adverse effect except mild diarrhea in the high dose of 2 g/kg b.w. of ethanolic extract [105]. Phytochemical studies on plant of G. tenax have shown the presence of triacontan-1-ol, α-amyrin, β-amyrin, β-Sitosterol, lupenne, erythrodiol, betulin, and tetratriacont-21-ol-12-one [4, 105].
3.15. Haloxylon salicornicum Moq
Haloxylon salicornicum Moq. (family: Chenopodiaceae) locally known as “Armas” is a stout herb with green succulent branches distributed in all the regions of Saudi Arabia [6]. In Arabian Peninsula and other Asian countries H. salicornicum has been used for the treatment of jaundice, gall bladder stones, liver diseases, digestive problems, inflammatory disorder, and joint diseases [106–108]. Experimental studies confirmed hepatoprotective [109], anti-inflammatory [110, 187], and antiulcer [107] activity of H. salicornicum.
The ethanolic extract of H. salicornicum dose dependently attenuated CCl4 induced increase in liver enzymes and histological changes [109]. Recently Alqasoumi et al. [110] reported antioxidant and anti-inflammatory activities of H. salicornicum which may contribute to its hepatoprotective activity. The toxicity studies on H. salicornicum extract showed that, even in the high dose of 4 g/kg b.w., the extract did not produce any symptoms of toxicity or death in rats [110, 188]. Phytochemical studies on aerial part of H. salicornicum has shown the presence of alkaloids, tannins, saponin glycosides, 7-hydroxy-4-triacontanone, 24-hydroxy-4-octacosanone, 1-triacontanol, β-amyrin, 24-ethylcholesta-3,5-diene, 24-nor-12-ursene, β-sitosterol, ursolic acid, and β-sitosterol [109–111].
3.16. Hypericum perforatum Linn
Hypericum perforatum Linn. (family: Hypericaceae) locally known as “Ashba berfortum” is perennial herbs/shrubs with yellow flower. It is popularly known as St John's wart. It is found in southern region of Saudi Arabia [6]. The medicinal use of herbs is mentioned in the writing of famous Greeco-Arab physicians Istikoglou et al. [189]. Avicina, a famous Arab physician in his book “Canon of medicine” (“Al-Quanoon fil Tib” in Arabic), also described medicinal properties of this herb [190]. The traditional medicinal uses of H. perforatum include treatment of jaundice, liver diseases, gall bladder stones, rheumatoid arthritis, and other inflammatory conditions [112–114].
Ozturk et al. [113] reported the hepatoprotective effect of alcoholic extract of aerial part of H. perforatum extract. The extract significantly attenuated CCI4 and ethanol [112, 113] induced hepatic toxicity. Experimental studies also showed significant choleretic activity of H. Perforatum [112]. The protective action of H. perforatum has been attributed to its anti-inflammatory [191], antioxidant, and immunomodulating activities [192]. Acute toxicity studies in rodent showed no toxicity; however chronic administration for 2 weeks showed significant signs of erythema, dermal edema, alopecia, and changes in blood chemistry. The animals gained less weight as compared to control in chronically treated groups [193]. Phytochemical studies on plant of H. perforatum showed the presence of hypericin, pseudohypericin, hyperforin, adhyperforin, quercetin, hyperoside, rutin, campferol, myricetin, amentoflavone, kielcorin, and norathyriol [115].
3.17. Juniperus procera Hochst. ex Endl
Juniperus procera Hochst. ex Endl. (family: Cupressaceae) locally known as “Arar” is a long tree with needle like leaves found in Hejaz and southern region of Saudi Arabia [6]. The plant has long been used in Saudi traditional medicine for liver disease, jaundice, digestive problems, and inflammatory diseases [116]. The resin of J. procera in combination with honey is also used as cure for liver diseases and ulcers [115].
The ethanolic extracts of aerial part of J. procera showed significant hepatoprotective activity against CCl4 induced liver injury [117]. The hepatoprotective activity has been attributed to terpene contents of J. procera [116]. J. procera possess significant antioxidant/free radical scavenging [194] and anti-inflammatory activities [195] which may contribute to its hepatoprotective activity. Acute and chronic toxicity studies revealed that the extract of J. procera is free from toxicity even in high dose [116]. Phytochemical studies on aerial part of J. procera showed the presence of terpenes, β-peltatin A, deoxypodophyllotoxin, and totarol [117].
3.18. Lepidium sativum Linn
Lepidium sativum Linn. (family: Cruciferae) locally known as “El-Rshad” is a fast-growing, edible herb with tangy flavour and aroma [6]. In traditional system of medicine various parts of plant have been used for the treatment of jaundice, liver problems, spleen diseases, gastrointestinal disorders, arthritis, and other inflammatory conditions [53, 118].
Hepatoprotective effect of methanolic extracts of L. sativum seeds was evaluated against CCl4 induced liver damage in rats. The extract dose dependently attenuated CCl4 induced rise in serum levels of AST, ALT, APT, and bilirubin suggesting its hepatoprotective activity [196]. Recently L. sativum has been shown to possess significant antioxidant [197–199] and anti-inflammatory [200] activities which may contribute to its hepatoprotective effect. In rats, up to 2% w/w of L. sativum in diet did not produce any toxicity, whereas 10% w/w showed mild toxicity [201]. Phytochemical studies on seed of L. sativum showed the presence of alkaloids, saponins, anthracene glycosides, carbohydrates, proteins, amino acids, flavonoids, and sterols [118].
3.19. Moringa oleifera Lam
Moringa oleifera Lam. (family: Moringaceae) locally known as “Ruwag” is a small, graceful, deciduous tree with sparse foliage [6]. The plant grows abundantly in many tropical and subtropical countries. Moringa is an ancient magic plant with a plethora of medicinal and nutritional value. The leaves, flowers, root, gums, fruit, and seed of M. oleifera have been extensively used in traditional medicine for the treatment of liver disease, lipid disorders, arthritis, and other inflammatory disorders [119–122].
Hepatoprotective effect of the ethanolic extract of M. oleifera leaves was studied against antitubercular drugs (isoniazid, rifampicin, and pyrazinamide) [202] induced liver damage as well as against cadmium induced hepatotoxicity in rats. Moringa extract significantly attenuated hepatotoxin induced biochemical (serum AST, ALT, APT, and bilirubin) and histopathological changes in liver. The hepatoprotective activity of M. oleifera was comparable with silymarin [203]. The extracts of M. oleifera leaves also showed significant antioxidant [204] and anti-inflammatory [120, 205] activities which may contribute to its hepatoprotective effect. The aqueous extract of M. oleifera is relatively safe with an LD50 value of 5 g/kg b.w. in mice [206]. Phytochemical studies of M. oleifera showed the presence of alkaloids, anthocyanins, β-carotene, protein, vitamin C, phenolics, calcium, iron, and potassium [122].
3.20. Nigella sativa Linn
Nigella sativa Linn. (family: Ranunculaceae) locally known as “Habbul-Barka” is a widely used medicinal plant throughout the world. According to Islamic and Arab literature, black seed of N. sativa is one of the most powerful herbal drugs used as liver tonics, digestive, anti-inflammatory, immunostimulant, and remedy for jaundice [123, 124].
Aqueous suspension of seeds powder of N. sativa showed significant hepatoprotective activity against CCl4 and ischemic-reperfusion induced liver injury [124, 207–211]. The anti-inflammatory [212–214] immunomodulating [215] antioxidant [216] activities of N. sativa may contribute to its hepatoprotective activity. The extracts and oil are relatively safe. The oral LD50 value of N. sativa fixed oil was found to be 28.8 mL/kg b.w. in mice [217]. Phytochemical studies on plant of N. sativa have shown the presence of thymoquinone, thymohydroquinone, dithymoquinone, p-cymene, carvacrol, and 4-terpineol [125].
3.21. Peganum harmala Linn
Peganum harmala Linn. (family: Nitrariaceae) locally known as “Harmal/Naqt” is a glabrous shrub found mostly in northern Hejaz and eastern Najd region of Saudi Arabia [6]. In traditional medicine P. harmala has been used for the treatment of jaundice, digestive disorders, liver diseases, and arthritis [126–129].
The hepatoprotective effect of ethanol and chloroform extracts of P. harmala seeds has been studied against thiourea [131] and CCl4 induced hepatotoxicity [218, 219] in rats. Both extracts dose dependently attenuated hepatotoxin induced biochemical (serum AST, ALT, and bilirubin) and histopathological changes suggesting its hepatoprotective activity. The extract also showed antioxidant [219] and anti-inflammatory [220] activities which may contribute to its hepatoprotective activity. Acute toxicity studies on the aqueous extract of P. harmala revealed that large doses may cause reversible tremors and convulsions in rats [221]. Oral LD50 in Wistar rats was found to be 2.70 g/kg b.w. In chronic studies aqueous extract of P. harmala administered orally for six weeks at doses of 1, 1.35, and 2 g/kg b.w. daily for 3-month period increased liver enzyme suggesting its hepatotoxicity. Histologic study also showed liver degeneration and spongiform changes in the central nervous system (CNS) in chronically treated rats [222]. Phytochemical studies on plant of P. harmala showed the presence of harmaline, harmine, harmalol, and tetrahydroharmine [130, 131, 223].
3.22. Pergularia daemia Forsk
Pergularia daemia Forsk. (family: Apocynaceae) locally known as “Ghalqa” is a climbing plant with thin glabrous leaves found in Najd region of Saudi Arabia [6]. In traditional system of medicine the whole aerial part of the plant is extensively used for the treatment of jaundice, liver diseases, and inflammatory disorders [132–134].
The ethanolic extracts of aerial parts of P. daemia dose dependently prevented the paracetamol [133] and CCl4 [134, 135] induced biochemical (serum AST, ALT, APT, and TB) and histopathological changes in the liver. Recent studies on P. daemia showed significant anti-inflammatory, antioxidant, and free radical scavenging activities [132–134] which may also contribute to its hepatoprotective activity. The ethanolic extract of P. daemia is relatively safe as it did not produce any toxicity up to a dose of 1.5 g/kg b.w. in mice [224]. Phytochemical studies on P. daemia have shown the presence of cardenolides, alkaloids, flavonoids, saponins, triterpenes, and steroidal compounds [134, 135].
3.23. Petroselinum crispum Mill
Petroselinum crispum Mill. (family: Umbelliferae) locally known as “Baqdunis” is a biennial herb widely grown in all the regions of Saudi Arabia [6]. P. crispum has been used in Arab traditional medicine, for the treatment of inflammatory condition, liver diseases, constipation, flatulence, jaundice, colic pain, and rheumatism [136, 137].
Ethanolic extract of P. crispum leaves has been pharmacologically investigated for its hepatoprotective activity [138]. The extract dose dependently attenuated CC14 induced increase in serum AST, ALT, ALP, and total bilirubin. The ethanolic extract of P. crispum leaves also showed significant anti-inflammatory [138] and antioxidant [225, 226] activities which may contribute to its hepatoprotective action. Although perfectly safe in pharmacological doses, P. crispum may be toxic in excess, especially when used as essential oil [227]. Phytochemical studies on P. crispum have showed the presence of flavones glycosides, apigenin-7-O-glucoside or cosmosiin, apigenin-7-O-apiosyl-O-glucoside/apiin, and the coumarin 2′′,3′′-dihydroxy furanocoumarin/oxypeucedanin hydrate [138–140].
3.24. Phyllanthus maderaspatensis Linn
Phyllanthus maderaspatensis Linn. (family: Euphorbiaceae) locally known as “Damabas” is a small branched shrub with scattered leaves and grows abundantly in eastern Najd and southern Hejaz region of Saudi Arabia [6]. In traditional medicine, sap and leaf decoction have been used as emetic and purgative; decoction of root is used for constipation, digestion, and abdominal pain. The aerial parts of plant have been used for treating liver disorders, rheumatism, and inflammatory diseases [141–143].
The hepatoprotective activity of whole plant extract of P. maderaspatensis has been investigated using several experimental models of hepatotoxicity [142, 228]. The extract significantly attenuated CCl4 induced biochemical (serum AST and ALT) and histopathological changes in liver. The hepatoprotective effect of Phyllanthus was comparable with silymarin [142]. P. maderaspatensis showed strong antioxidant [229] and anti-inflammatory [230] activities which may contribute to its hepatoprotective activity. P. maderaspatensis is considered as safe in pharmacological doses [231]. Phytochemical studies on P. maderaspatensis showed the presence of carbohydrates, proteins, flavonoids, essential oil, and tannins. Seeds of P. maderaspatensis contain long chain fatty acids and β-sitosterol [142]. Defatted seed cake contains mucilage, which yields galactose, arabinose, rhamnose and aldobionic acid, niruriside, phyllanthin, hypophyllanthin, and cinnamoyl sucrose acetate [144].
3.25. Pimpinella anisum Linn
Pimpinella anisum Linn. (family: Umbelliferae) locally known as “Alyansoon” is one of the oldest known annual medicinal herbs with white flowers and small seeds. In Arab traditional medicine the plant is used as digestive, carminative, antispasmodic, and liver disorders [145, 146].
Diethyl ether extract of P. anisum seed has been investigated for its hepatoprotective activity in rats. The extract dose dependently attenuated CCl4 induced rise liver enzymes including AST and ALT [232]. P. anisum possess significant antioxidant [233, 234] and anti-inflammatory [235] activities which may contribute to its hepatoprotective efficacy. Oral lethal dose of anise oil in human being ranges between 50 and 5000 mg/kg [236]. Essential oil of P. anisum has an LD50 value of 0.84 mL/kg b.w of mice whereas the fixed oil has an LD50 value of 3.15 mL/kg in mice [237]. Phytochemical studies on plant of P. anisum have shown the presence of volatile oils (anethole, eugenol, methyl chavicol, and estragole), fatty acids (palmitic, petroselinic, vaccenic, and oleic acids), and coumarins [147].
3.26. Portulaca oleracea Linn
Portulaca oleracea Linn. (family: Portulacaceae) locally known as “Rizlah” and “Farfahena” is an annual herb with branched stems found in Hejaz region and eastern part of Saudi Arabia [6]. The medicinal use of P. oleracea was known by Arabs from the time of Pharaohs [238]. It is used for the treatment of liver disorders, gastrointestinal problems, and inflammatory condition [82, 148].
The hepatoprotective activity of the aqueous and ethanolic extract of P. oleracea whole plant has been investigated by several investigators [148, 149, 239]. The extract significantly attenuated CCl4 induced rise in biochemical (serum AST, APT, TB, and total protein) and histopathological changes in liver. It also antagonised CCl4 and prolonged pentobarbitone induced sleeping time clearly suggesting significant hepatoprotective activity. The extracts of P. oleracea also showed significant antioxidant [240] and anti-inflammatory [241] activities which may contribute to its hepatoprotective activity. Methanolic extract of P. oleracea has an LD50 value of 1.8 g/kg b.w. in mice. In high doses the extract may cause kidney, lung, and liver toxicity in a dose dependent manner [242]. P. oleracea contains several biologically active compounds that include, alkaloids, coumarins, flavonoids, cardiac glycosides, anthraquinone glycosides, alanine, saponins, tannins, and organic acids (free oxalic acids, cinnamic acids, caffeic acid, malic acids, and citric acids). Omega-3-acids, alpha-linolenic acid, vitamins, glutathione, glutamic acid, and aspartic acid containing β-sitosterol have also been found in various parts of plants [149–151].
3.27. Rhazya stricta Decne
Rhazya stricta Decne. (family: Apocynaceae) locally known as “harmal” is a perennial sand binding under shrub found in all regions of Saudi Arabia [6]. In the honor of Al-Rhazes, a leading scholar and physician of Arab and Islamic world, the plant was named as Rhazya stricta. In traditional medicine the plant is used for the treatment of inflammatory condition, stomach problems, and liver diseases [152–154].
Pretreatment with R. stricta significantly protected mice against paracetamol induced biochemical changes and prolongation of pentobarbitone induced sleeping time. The hepatoprotective effect of R. stricta was comparable with silymarin [36]. The extract of R. stricta leaves also showed significant antioxidant [154] and anti-inflammatory [243] activities which may contribute to its hepatoprotective activity. Ingestion in therapeutic doses is perfectly safe in human; however chronic administration of high doses in rats has shown variety of toxic effects including decrease in growth rate, dullness, and hepatonephrotoxicity [155, 244]. Phytochemical studies on R. stricta showed the presence of alkaloids (rhazimine, stemmadenine, vincadine, and rhazimanine), carboline, and flavonoidal glycoside [36, 154, 155].
3.28. Smilax regelii Killip and CV Morton
Smilax regelii Killip and CV Morton (family: Liliaceae) locally known as “Nabatul Fusaq” is a perennial, trailing vine with prickly stems [6]. The plant commonly known as sarsaparilla has been widely used for the treatment of liver diseases, arthritis, and other inflammatory conditions and as an immunomodulator in Greeko-Arab system of medicine [156–158]. Besides its medicinal use, sarsaparilla is often used as a flavouring agent in nonalcoholic drinks [245]. A decoction made from the roots is used as a vehicle in the preparation of syrups which have been reported to have cooling properties [246].
The hepatoprotective effect of the ethanol extract of roots of S. regelii has been studied in rats. Ethanolic extract of sarsaparilla significantly inhibited CCI4 induced rise in AST, ALT, and bilirubin, in serum in rats [247]. The extract showed strong antioxidant [247], anti-inflammatory [159], and immunomodulating [248] activities which may contribute to its hepatoprotective property. No known toxicity or side effects have been documented for sarsaparilla; however ingestion of large doses may cause gastric irritation [160]. Phytochemical studies on plant of S. regelii showed the presence of cetyl-parigenin, astilbin, beta-sitosterol, caffeoyl-shikimic acids, dihydroquercetin, diosgenin, engeletin, essential oils, epsilon-sitosterol, eucryphin, eurryphin, ferulic acid, glucopyranosides, isoastilbin, isoengetitin, kaempferol, parigenin, parillin, pollinastanol, resveratrol, rhamnose, saponin, sarasaponin, sarsaparilloside, sarsaponin, sarsasapogenin, shikimic acid, sitosterol-d-glucoside, smilagenin, smilasaponin, smilax saponins A-C, smiglaside A-E, smitilbin, stigmasterol and taxifolin, and titogenin [159, 160].
3.29. Solanum nigrum Linn
Solanum nigrum Linn. (family: Solanaceae) locally known as “Anaab ud dib” is an annual hairy herb with ovate to oblong leaves abundant in all parts of Saudi Arabia [6]. The plant is a house hold remedy for liver disorders, jaundice and cirrhosis, inflammatory condition, rheumatism, and swollen joints [161–163].
The extracts of whole plant of S. nigrum significantly attenuated CCl4 [164, 165, 249–251] and thioacetamide [252] induced biochemical (serum AST, ALT, APT, and TB) and histopathological changes in liver. The hepatoprotective action of S. nigrum may be attributed to its antioxidant [253] and anti-inflammatory [254] constituents. LD50 value of ethanol extract of the fruits of S. nigrum in rats was found to be 2 g/kg b.w. [255]. Phytochemical studies on S. nigrum showed the presence of glycoalkaloids, glycoproteins, polysaccharides, gallic acid, catechin, protocatechuic acid, caffeic acid, epicatechin, rutin, and naringenin [164, 165].
3.30. Suaeda maritima Linn
Suaeda maritima Linn. (family: Amaranthaceae) locally known as “Sawad” is shrubs with continuous unjoined stems found in western region of Saudi Arabia [6, 256]. The juice of this herb is used for treatment of liver diseases by Arab practitioners [257]. The leaves are also used as remedy for liver, heart, and lipid disorders [166].
The ethanolic extracts of S. maritima leaves significantly attenuated concanavalin (a hepatotoxin) induced biochemical (serum AST, ALT, APT, and bilirubin) and histopathological changes in liver [167]. The extract of plant also showed significant antioxidant, anti-inflammatory, antiviral, and antibacterial activities [167, 168] which may contribute to its hepatoprotective activity. It is nontoxic edible plant which is used in salad and as fodder for animals [258]. The LD50 of ethanolic extract of S. maritima in rats was found to be 3 g/kg b.w. [167]. Phytochemical studies on plant of S. maritima showed the presence of alkaloid, flavonoid, sterols, phenolic compounds, and tannins [166–168].
3.31. Tamarix nilotica Ehrenb Bunge
Tamarix nilotica Ehrenb Bunge (family: Tamaricaceae) locally known as “Tarafa” is a green shrub with free distinct blade type leaves found in eastern Najd and northern region of Saudi Arabia [6]. Avicenna has mentioned this plant in his famous book “Canon of medicine” for the treatment of liver, stomach, and inflammatory problems [169–171].
The hydroalcoholic extract of T. nilotica flower showed marked hepatoprotective activity against CCl4 induced liver injury [171]. Experimental studies also showed highly significant antioxidant [171] and anti-inflammatory [259] activities of T. nilotica which may contribute its hepatoprotective activity. No experimental and clinical toxicity of T. nilotica has been reported. However plant possesses significant cytotoxicity against some human cancer cell lines [170]. Phytochemical studies on T. nilotica showed the presence of flavonoids, tannins, syringaresinol, isoferulic acid, niloticol, 3-hydroxy-4-methoxycinnamaldehyde, methyl and ethyl esters of gallic acid, para-methoxygallic acid, kaempferol, quercetin 3-oglucuronides, 3-o-sulphated kaempferol, 7,4′-dimethyl ether, and free flavonols [170, 171].
3.32. Tephrosia purpurea Linn
Tephrosia purpurea Linn. (family: Fabaceae) locally known as “Ami” is a perennial plant with imparipinnate leaves and grows in southern Hejaz region of Saudi Arabia [6]. T. purpurea has been used for centuries in traditional system of medicine for the treatment of jaundice, liver, biliary and splenic disease, and inflammatory disorders [172–174].
The hydroalcoholic extract of aerial parts of T. purpurea attenuated thioacetamide [175] induced hepatotoxicity in a dose dependent manner suggesting its significant hepatoprotective activity. The extract also showed antioxidant [175] and anti-inflammatory [260] activities, which may contribute to its hepatoprotective activity. T. purpurea is well tolerated in rats and produces no toxicity up to the dose of 2000 mg/kg b.w. Chronic administration of T. purpurea at doses of 200 and 400 mg/kg b.w. was also found safe in rats [261]. Phytochemical studies on T. purpurea have shown the presence of β-sitosterol, quercetin, lupeol, rutin, delphinidin chloride, cyanidin chloride, isolonchocarpin, lanceolatins A and B, pongamol, karangin, kangone, 5,7-dimethoxy-8-flavanone, and 2-methoxy-3,9-dihydroxycoumestone [175, 176].
3.33. Teucrium polium Linn
Teucrium polium Linn. (family: Lamiaceae) locally known as “Jaad” is a perennial branched shrub found in northern region, Nefud region, southern Hejaz, East Najd, and eastern province of Saudi Arabia [6]. T. polium is widely used by the folk-medicine practitioners of Saudi Arabia for the treatment of liver diseases, inflammatory disorders, stomach and intestinal troubles, and rheumatism [177].
The hydroalcoholic extract of aerial part of T. polium dose dependently attenuated CCl4 [178] and acetaminophen [262] induced biochemical (serum ALT, AST, APT, and total bilirubin) and histological changes in liver. Experimental studies of T. polium on cultured hepatocytes also confirmed its strong antioxidant [178, 263] and anti-inflammatory activities [177, 264, 265] which may contribute to its hepatoprotective activity. There is no report on acute toxicity of plants. However chronic administration of high dose of T. polium rats showed mild toxicity [266]. One case of severe hepatotoxicity has been reported in a patient following prolonged use of T. polium [267, 268]. Phytochemical studies on T. polium showed the presence of flavonoids, terpenes including syrapoline, thujene, caryophyllene, cedrol, epi-cadinol, and bisabolene [178–181].
3.34. Trianthema portulacastrum Linn
T. portulacastrum Linn. (family: Aizoaceae) locally known as “Laani” is a fleshy herb with opposite petiolated unequal leaves found in eastern province and southern Hejaz part of Saudi Arabia [6]. T. portulacastrum is widely used in Arab countries [269], Africa, India, and southeast Asia, for the treatment of jaundice, liver disorders, stomach problem, arthritis, and inflammation [182, 270, 271]. Laboratory investigations on extracts of the plant have demonstrated significant hepatoprotective, antioxidant, diuretic, analgesic, and anticarcinogenic activity [182].
The ethanolic extract of leaves of T. portulacastrum significantly attenuated the paracetamol [272], thioacetamide [272], and aflatoxin B [183] induced hepatotoxicity in experimental studies. The extract of T. portulacastrum also showed significant antioxidant [273, 274] and anti-inflammatory [275] activities, which may contribute to its hepatoprotective effect. Acute toxicity studies in albino mice suggested that the extract of T. portulacastrum was safe even at the dose of 3 g/kg b.w. [276]. Phytochemical studies on T. portulacastrum showed the presence of steroids, saponins, flavonoid, coumarins, terpenes, glycosides, tannins, alkaloids, and volatile oil [182, 183].
3.35. Tribulus terrestris Linn
Tribulus terrestris Linn. (family: Zygophyllaceae) locally known as “Darisa” is an annual procumbent herb with compound paripinnate leaves found in eastern Najd and southern Hejaz region of Saudi Arabia [6]. Local bedouin use the plant to treat urinary disorders, impotency, and liver diseases. The seeds of this plant are recommended in hemorrhages, kidney stone, and gout. The fruit is regarded as tonic, diuretic, and aphrodisiac [184].
The aqueous and hydroalcoholic extracts of fruit of T. terrestris dose dependently attenuated paracetamol [277] and ferrous sulphate [278] induced liver damage. Two compounds (tribulusamides A and B), isolated from the fruits of T. terrestris significantly, protected cultured hepatocytes against D-galactosamine induced toxicity [279]. T. terrestris has also been reported to possess antioxidant [280] and anti-inflammatory [281] activities which may contribute to its hepatoprotection. According to some reports grazing on T. terrestris caused hepatorenal syndrome and neurotoxicity in goats and sheep [282, 283]. Nephrotoxicity has also been reported in patient following chronic use of T. terrestris [284]. The extract also showed antispasmodic activity in rats [285]. Phytochemical studies on T. terrestris showed the presence of Tribulusamides A and B, tigogenin, neotigogenin, terrestrosid F, and gitonin [185].
4. Conclusion
Ancient classical literature and ethnomedical survey among local population clearly suggest that herbal drugs have been extensively used in Arab traditional medicine for the treatment of liver diseases. In this review we present the scientific appraisal of 35 herbal drugs used in Saudi traditional medicine for the treatment of liver disorders. The effect of herbs against hepatotoxin induced liver injury (based on biochemical markers and histopathological findings) has been summarized. Besides reviewing hepatoprotective efficacy and possible mechanism of action of these plant drugs, the available data on phytochemical constituents and their toxic untoward effects have been presented. Although the meta-analysis of available scientific literature on hepatoprotective activity of the herbs to a great extent substantiates folkloric claims about the usefulness of these botanicals to treat chronic liver diseases, the data regarding randomized clinical trials, safety studies, and quality control of these herbs is far from satisfactory.
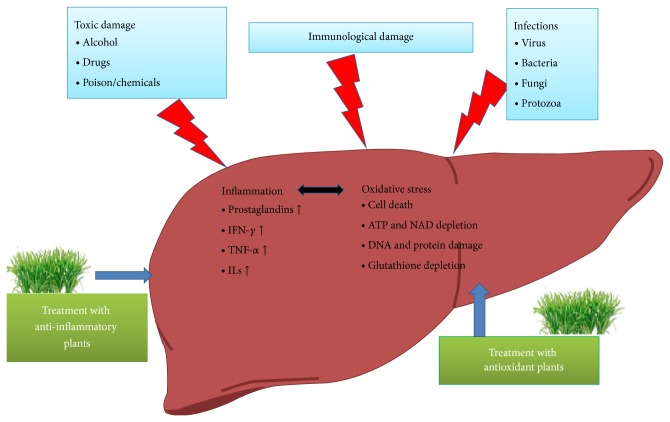
One of the noteworthy findings in this review is that the majority of hepatoprotective plants showed antioxidant and anti-inflammatory activities. The mechanism of hepatic injury invariably involves peroxidation of hepatocyte membrane fatty acids causing destruction of the cells and their intracellular organelles. According to the recent reports oxidative stress plays a pivotal role in the initiation and progression of hepatic damage following insult to a variety of hepatotoxins. The role of oxidative stress in viral hepatitis and autoimmune related liver diseases has been extensively documented. Moreover hepatotoxic chemicals damage liver cells primarily by producing reactive species which form covalent bond with the lipid moiety of the hepatic cell membranes (Figure 1).
Figure 1.
Anti-inflammatory and anti-oxidant herbs protect liver against variety of toxins and injurious stimuli by restoring the oxidative stress related liver damage and inflammatory cytokines.
Due to extensive exposure to hazardous chemicals, sometimes the free radicals generated are so high that they overpower the natural defensive system leading to hepatic damage. The drugs/chemicals with antioxidant properties such as Vitamin E and silymarin have been shown to protect against toxin induced hepatotoxicity. On the other hand inflammation is a key event in hepatotoxin induced liver damage. The toxins directly or through oxidative stress mechanism may trigger inflammatory response in the liver, which is evident from a significant increase in the proinflammatory cytokines including TNFα and IL6 and hepatocyte inflammation. Majority of hepatoprotective herbs have been shown to suppress oxidative stress and inflammation.
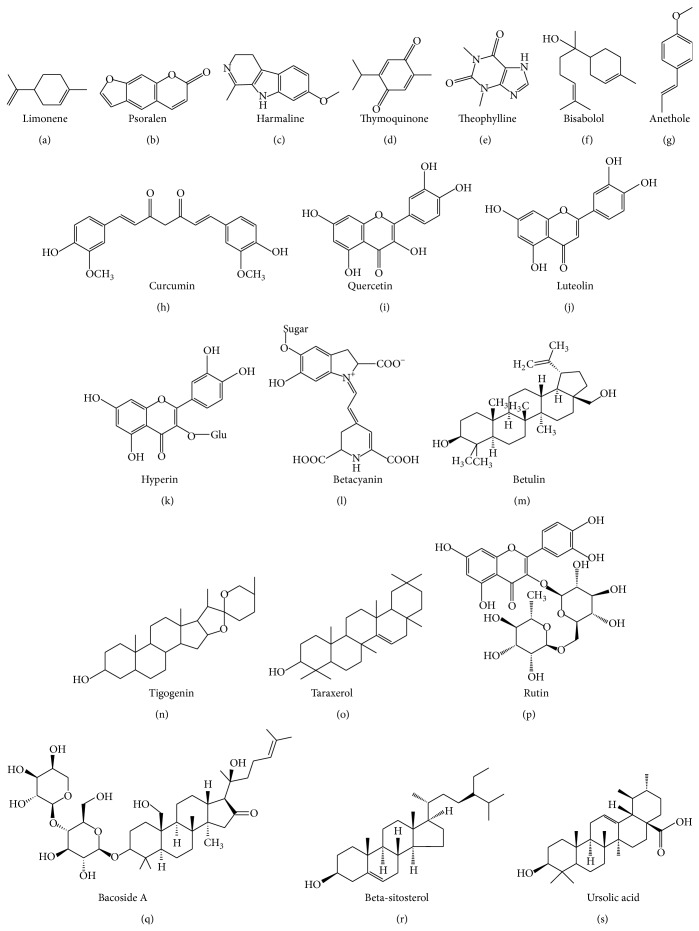
Our survey and published reports clearly suggest that medical plants used in traditional medicine are rich sources of medicinally active chemical constituents such as phenols, coumarins, lignans, terpenoids, carotenoids, glycosides, flavonoids, organic acids, alkaloids, and xanthene. Some of the purified phytomolecules isolated from these plants have also been shown to possess potent hepatoprotective activity (Figure 2).
Figure 2.
Chemical structure of some of the hepatoprotective phytoconstituents.
Further investigation into the lead molecules that may produce better, safe, and effective therapeutic effects is warranted to overcome the pharmaceutical imbalance between remedies that protect the liver and drugs that induce hepatotoxicity. Moreover quality control of herbal drugs and randomized controlled clinical trials will further validate the evidenced based herbal therapy for the treatment of liver diseases.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Subbarayappa B. V. The roots of ancient medicine: an historical outline. Journal of Biosciences. 2001;26(2):135–143. doi: 10.1007/BF02703637. [DOI] [PubMed] [Google Scholar]
- 2.Saad B., Azaizeh H., Said O. Arab herbal medicine. Botanical Medicine in Clinical Practice. 2008;4:31–39. [Google Scholar]
- 3.Saad B., Azaizeh H., Said O. Tradition and perspectives of Arab herbal medicine: a review. Evidence-Based Complementary and Alternative Medicine. 2005;2(4):475–479. doi: 10.1093/ecam/neh133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma N., Patni V. Grewia tenax (Frosk.) Fiori.—a traditional medicinal plant with enormous economic prospectives. Asian Journal of Pharmaceutical and Clinical Research. 2012;5(3):28–32. [Google Scholar]
- 5.Girish C., Pradhan S. C. Indian herbal medicines in the treatment of liver diseases: problems and promises. Fundamental and Clinical Pharmacology. 2012;26(2):180–189. doi: 10.1111/j.1472-8206.2011.01011.x. [DOI] [PubMed] [Google Scholar]
- 6.Migahid A. M. Flora of Saudi Arabia. 2nd. Riyadh, Saudi Arabia: Riyadh University; 1978. [Google Scholar]
- 7.Phillips H. History of Cultivated Vegetables. London, UK: Henry Colburn; 1827. [Google Scholar]
- 8.Karim A., Bhatty M. K. Studies on the essential oils of the Pakistani species of the family Umbelliferae. IV. Apium graveolens Linn. (celery, ajmodn) seed oil. Pakistan Journal of Scientific and Industrial Research. 1976;19:243–246. [Google Scholar]
- 9.Guenther E. The Essential Oils. New York, NY, USA: Van Nostrand Reinhold; 1950. [Google Scholar]
- 10.Ahmed B., Alam T., Varshney M., Khan S. A. Hepatoprotective activity of two plants belonging to the Apiaceae and the Euphorbiaceae family. Journal of Ethnopharmacology. 2002;79(3):313–316. doi: 10.1016/S0378-8741(01)00392-0. [DOI] [PubMed] [Google Scholar]
- 11.Singh A., Handa S. S. Hepatoprotective activity of Apium graveolens and Hygrophila auriculata against paracetamol and thioacetamide intoxication in rats. Journal of Ethnopharmacology. 1995;49(3):119–126. doi: 10.1016/0378-8741(95)01291-5. [DOI] [PubMed] [Google Scholar]
- 12.Popović M., Kaurinović B., Trivić S., Mimica-Dukić N., Bursać M. Effect of celery (Apium graveolens) extracts on some biochemical parameters of oxidative stress in mice treated with carbon tetrachloride. Phytotherapy Research. 2006;20(7):531–537. doi: 10.1002/ptr.1871. [DOI] [PubMed] [Google Scholar]
- 13.Al-Howiriny T., Alsheikh A., Alqasoumi S., Al-Yahya M., Eltahir K., Rafatullah S. Gastric antiulcer, antisecretory and cytoprotective properties of celery (Apium graveolens) in rats. Pharmaceutical Biology. 2010;48(7):786–793. doi: 10.3109/13880200903280026. [DOI] [PubMed] [Google Scholar]
- 14.Atta A. H., Alkofahi A. Anti-nociceptive and anti-inflammatory effects of some Jordanian medicinal plant extracts. Journal of Ethnopharmacology. 1998;60(2):117–124. doi: 10.1016/S0378-8741(97)00137-2. [DOI] [PubMed] [Google Scholar]
- 15.Momin R. A., Nair M. G. Antioxidant, cyclooxygenase and topoisomerase inhibitory compounds from Apium graveolens Linn. seeds. Phytomedicine. 2002;9(4):312–318. doi: 10.1078/0944-7113-00131. [DOI] [PubMed] [Google Scholar]
- 16.Perry L. M. Medicinal Plants of East and South East Asia. London, UK: The MIT Press; 1980. [Google Scholar]
- 17.Sarin R., Singh A. Artemisinin content in Artemisia scoparia . Recent Research in Science and Technology. 2010;2(6):47–50. [Google Scholar]
- 18.Gilani A. H., Janbaz K. H. Hepatoprotective effects of Artemisia scoparia against carbon tetrachloride: an environmental contaminant. Journal of the Pakistan Medical Association. 1994;44(3):65–68. [PubMed] [Google Scholar]
- 19.Noguchi T., Lai E. K., Alexander S. S., King M. M., Olson L., Lee Poyer J., McCay P. B. Specificity of a phenobarbital-induced cytochrome P-450 for metabolism of carbon tetrachloride to the trichloromethyl radical. Biochemical Pharmacology. 1982;31(5):615–624. doi: 10.1016/0006-2952(82)90440-3. [DOI] [PubMed] [Google Scholar]
- 20.Gilani A.-U. H., Janbaz K. H. Protective effect of Artemisia scoparia extract against acetaminophen-induced hepatotoxicity. General Pharmacology. 1993;24(6):1455–1458. doi: 10.1016/0306-3623(93)90434-Y. [DOI] [PubMed] [Google Scholar]
- 21.Liu C.-X., Ye G.-Z. Choleretic activity of p-hydroxyacetophenone isolated from Artemisia scoparia Waldst. et Kit. in the rat. Phytotherapy Research. 1991;5(4):182–184. doi: 10.1002/ptr.2650050410. [DOI] [Google Scholar]
- 22.Habib M., Waheed I. Evaluation of anti-nociceptive, anti-inflammatory and antipyretic activities of Artemisia scoparia hydromethanolic extract. Journal of Ethnopharmacology. 2013;145(1):18–24. doi: 10.1016/j.jep.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 23.Singh H. P., Mittal S., Kaur S., Batish D. R., Kohli R. K. Chemical composition and antioxidant activity of essential oil from residues of Artemisia scoparia . Food Chemistry. 2009;114(2):642–645. doi: 10.1016/j.foodchem.2008.09.101. [DOI] [Google Scholar]
- 24.Negahban M., Moharramipour S., Yousefelahi M. Efficacy of essential oil from Artemisia scoparia Waldst. & Kit. against Tribolium castaneum (Herbst) (Coleoptera:Tenebrionidae). Proceedings of the 4th International Iran & Russia Conference, Agricultural and Natural Resources; September 2004; Shahrekord, Iran. [Google Scholar]
- 25.Anonymous Artemisia scoparia, http://www.naturalmedicinalherbs.net/herbs/a/artemisia-scoparia.php.
- 26.Lin S., Xiao Y.-Q., Zhang Q.-W., Zhang N.-N. Studies on chemical constituents in bud of Artemisia scoparia (II) China Journal of Chinese Materia Medica. 2004;29(2):152–154. [PubMed] [Google Scholar]
- 27.Bammidi S. R., Volluri S. S., Chippada S. C., Avanigadda S., Vangalapati M. A review on pharmacological studies of Bacopa monniera . Journal of Chemical, Biological and Physical Sciences. 2011;1(2):250–259. [Google Scholar]
- 28.Anonymous Brahmi: “Herbs of Grace”, http://www.ayurvedacollege.com/articles/students/Brahmi.
- 29.Menon B. R., Rathi M. A., Thirumoorthi L., Gopalakrishnan V. K. Potential effect of Bacopa monniera on nitrobenzene induced liver damage in rats. Indian Journal of Clinical Biochemistry. 2010;25(4):401–404. doi: 10.1007/s12291-010-0048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sumathy T., Subramanian S., Govindasamy S., Balakrishna K., Veluchamy G. Protective role of Bacopa monniera on morphine induced hepatotoxicity in rats. Phytotherapy Research. 2001;15(7):643–645. doi: 10.1002/ptr.1007. [DOI] [PubMed] [Google Scholar]
- 31.Channa S., Dar A., Anjum S., Yaqoob M. Anti-inflammatory activity of Bacopa monniera in rodents. Journal of Ethnopharmacology. 2006;104(1-2):286–289. doi: 10.1016/j.jep.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 32.Joshua Allan J., Damodaran A., Deshmukh N. S., Goudar K. S., Amit A. Safety evaluation of a standardized phytochemical composition extracted from Bacopa monniera in Sprague-Dawley rats. Food and Chemical Toxicology. 2007;45(10):1928–1937. doi: 10.1016/j.fct.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 33.Weissner W. Brahmi and Cognition: Nature’s Brainpower Enhancer. http://ayurveda-nama.org/pdf/resources/NAMA_Brahmi_Weissner.pdf.
- 34.Sudharani D., Krishna K. L., Deval K., Safia A. K. Pharmacological profiles of Bacopa monnieri: a review. International Journal of Pharmacy. 2011;1(1):15–23. [Google Scholar]
- 35.Yadav J., Panghal M. Balanites aegyptiaca (L.) Del. (Hingot): a review of its traditional uses, phytochemistry and pharmacological properties. International Journal of Green Pharmacy. 2010;4(3):140–146. doi: 10.4103/0973-8258.69158. [DOI] [Google Scholar]
- 36.Ali B. H., Bashir A. K., Rasheed R. A. Effect of the traditional medicinal plants Rhazya stricta, Balanitis aegyptiaca and Haplophylum tuberculatum on paracetamol-induced hepatotoxicity in mice. Phytotherapy Research. 2001;15(7):598–603. doi: 10.1002/ptr.818. [DOI] [PubMed] [Google Scholar]
- 37.Jaiprakash B., Aland R., Karadi R. V., Savadi R. V., Hukkeri V. L. Heptoprotective activity of bark of Balanites aegyptiaca Linn. Journal of Natural Remedies. 2003;3(2):205–207. [Google Scholar]
- 38.Suky T. M. G., Parthipan B., Kingston C., Mohanand P. V. R., Soris T. Hepatoprotective and antioxidant effect of Balanites aegyptiaca (L.) Del against CCl4 induced hepatotoxicity in rats. International Journal of Pharma Sciences and Research. 2011;2(4):887–892. [Google Scholar]
- 39.Gaur K., Nema R. K., Kori M. L., Sharma C. S., Singh V. Anti-inflammatory and analgesic activity of Balanites aegyptiaca in experimental animal models. International Journal of Green Pharmacy. 2008;2(4):214–217. [Google Scholar]
- 40.Obidah W., Nadro M. S., Tiyafo G. O., Wurochekke A. U. Toxicity of crude Balanites aegyptiaca seed oil in rats. Journal of American Science. 2009;5:13–16. [Google Scholar]
- 41.Chothani D. L., Vaghasiya H. U. A review on Balanites aegyptiaca Del (desert date): phytochemical constituents, traditional uses, and pharmacological activity. Pharmacognosy Reviews. 2011;5(9):55–62. doi: 10.4103/0973-7847.79100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jain N. K., Singhai A. K. Protective role of Beta vulgaris L. leaves extract and fractions on ethanol-mediated hepatic toxicity. Acta Poloniae Pharmaceutica—Drug Research. 2012;69(5):945–950. [PubMed] [Google Scholar]
- 43.Kirtikar K. R., Basu B. D. Text Book of Indian Medicinal Plants. Allahabad, India: Lalit Mohan Basu; 2005. [Google Scholar]
- 44.Chopra R. N., Nayar S. L., Chopra I. C. Glossary of Indian Medicinal Plants. New Delhi, India: CSIR; 1956. [Google Scholar]
- 45.Agarwal M., Srivastava V. K., Saxena K. K., Kumar A. Hepatoprotective activity of Beta vulgaris against CCl4-induced hepatic injury in rats. Fitoterapia. 2006;77(2):91–93. doi: 10.1016/j.fitote.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Georgiev V. G., Weber J., Kneschke E.-M., Denev P. N., Bley T., Pavlov A. I. Antioxidant activity and phenolic content of betalain extracts from intact plants and hairy root cultures of the red beetroot Beta vulgaris cv. Detroit Dark Red. Plant Foods for Human Nutrition. 2010;65(2):105–111. doi: 10.1007/s11130-010-0156-6. [DOI] [PubMed] [Google Scholar]
- 47.Chakole R., Zade S., Charde M. Antioxidant and anti-inflammatory activity of ethanolic extract of Beta vulgaris Linn. roots. International Journal of Biomedical and Advance Research. 2011;2(4):124–130. [Google Scholar]
- 48.Rajpoot K., Mishra R. N. Boerhaavia diffusa roots (Punarnava mool)—review as rasayan (rejuvenator/antiaging) International Journal of Pharmaceutical and Biomedical Research. 2011;2(4):1451–1460. [Google Scholar]
- 49.Olaleye M. T., Akinmoladun A. C., Ogunboye A. A., Akindahunsi A. A. Antioxidant activity and hepatoprotective property of leaf extracts of Boerhaavia diffusa Linn. against acetaminophen-induced liver damage in rats. Food and Chemical Toxicology. 2010;48(8-9):2200–2205. doi: 10.1016/j.fct.2010.05.047. [DOI] [PubMed] [Google Scholar]
- 50.Devaki T., Shivashangari K. S., Ravikumar V., Govindaraju P. Hepatoprotective activity Boerhaavia diffusa on ethanol-induced liver damage in rats. Journal of Natural Remedies. 2004;4(2):109–115. [Google Scholar]
- 51.Bhalla T. N., Gupta M. B., Sheth P. K., Bhargava K. P. Antiinflammatory activity of Boerhaavia diffusa . Indian Journal of Physiology and Pharmacology. 1968;6(1):11–16. [Google Scholar]
- 52.Orisakwe O. E., Afonne O. J., Chude M. A., Obi E., Dioka C. E. Sub-chronic toxicity studies of the aqueous extract of Boerhavia diffusa leaves. Journal of Health Science. 2003;49(6):444–447. doi: 10.1248/jhs.49.444. [DOI] [Google Scholar]
- 53.Al-Zahim A. A., Al-Malki N. Y., Al-Abdulkarim F. M., Al-Sofayan S. A., Abunab H. A., Abdo A. A. Use of alternative medicine by Saudi liver disease patients attending a tertiary care center: prevalence and attitudes. Saudi Journal of Gastroenterology. 2013;19(2):75–80. doi: 10.4103/1319-3767.108477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chantre P., Lairon D. Recent findings of green tea extract AR25 (exolise) and its activity for the treatment of obesity. Phytomedicine. 2002;9(1):3–8. doi: 10.1078/0944-7113-00078. [DOI] [PubMed] [Google Scholar]
- 55.Sengottuvelu S., Duraisami S., Nandhakumar J., Duraisami R., Vasudevan M. Hepatoprotective activity of Camellia sinensis and its possible mechanism of action. Iranian Journal of Pharmacology and Therapeutics. 2008;7(1):9–14. [Google Scholar]
- 56.Ratnasooriya W. D., Fernando T. S. P. Gastric ulcer healing activity of Sri Lankan black tea (Camellia sinensis L.) in rats. Pharmacognosy Magazine. 2009;1(1):11–20. [Google Scholar]
- 57.Nag Chaudhuri A. K., Karmakar S., Roy D., Pal S., Pal M., Sen T. Anti-inflammatory activity of Indian black tea (Sikkim variety) Pharmacological Research. 2005;51(2):169–175. doi: 10.1016/j.phrs.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 58.Sur P., Chaudhuri T., Vedasiromoni J. R., Gomes A., Ganguly D. K. Antiinflammatory and antioxidant property of saponins of tea [Camellia sinensis (L.) O. Kuntze] root extract. Phytotherapy Research. 2001;15(2):174–176. doi: 10.1002/ptr.696. [DOI] [PubMed] [Google Scholar]
- 59.Gomes A., Das M., Vedasiromoni J. R., Ganguly D. K. Proconvulsive effect of tea (Camellia sinensis) in mice. Phytotherapy Research. 1999;13(5):376–379. doi: 10.1002/(sici)1099-1573(199908/09)13:5<376::aid-ptr465>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 60.Pedrós C., Cereza G., García N., Laporte J.-R. Liver toxicity of Camellia sinensis dried atanolic extract. Medicina Clinica. 2003;121(15):598–599. doi: 10.1157/13053801. [DOI] [PubMed] [Google Scholar]
- 61.Bonkovsky Md H. L. Hepatotoxicity associated with supplements containing Chinese green tea (Camellia sinensis) Annals of Internal Medicine. 2006;144(1):68–71. doi: 10.7326/0003-4819-144-1-200601030-00020. [DOI] [PubMed] [Google Scholar]
- 62.Dias T. R., Tomás G., Teixeira N. F., Alves M. G., Oliveira P. F., Silva B. M. White tea (Camellia sinensis (L.)): antioxidant properties and beneficial health effects. International Journal of Food Science, Nutrition and Dietetics. 2013;2(2):1–15. [Google Scholar]
- 63.Anonymous . Indian Medicinal Plants. Madras, India: Orient Longman; 1995. [Google Scholar]
- 64.Schuppan D., Jia J. I.-D., Brinkhaus B., Hahn E. G. Herbal products for liver diseases: a therapeutic challenge for the new millennium. Hepatology. 1999;30(4):1099–1104. doi: 10.1002/hep.510300437. [DOI] [PubMed] [Google Scholar]
- 65.Solanki Y. B., Jain S. M. Hepatoprotective effects of Clitoria ternatea and Vigna mungo against acetaminophen and carbon tetrachloride-induced hepatotoxicity in rats. Journal of Pharmacology and Toxicology. 2011;6(1):30–48. doi: 10.3923/jpt.2011.30.48. [DOI] [Google Scholar]
- 66.Nithianantham K., Shyamala M., Chen Y., Latha L. Y., Jothy S. L., Sasidharan S. Hepatoprotective potential of Clitoria ternatea leaf extract against paracetamol induced damage in mice. Molecules. 2011;16(12):10134–10145. doi: 10.3390/molecules161210134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Narayanasamyd K., Selvi V. Hepatoprotective effect of a polyherbal formulation (ayush-liv. 04) against ethanol and CCl4 induced liver damage in rats. Ancient Science of Life. 2005;25(1):28–33. [PMC free article] [PubMed] [Google Scholar]
- 68.Devi B. P., Boominathan R., Mandal S. C. Anti-inflammatory, analgesic and antipyretic properties of Clitoria ternatea root. Fitoterapia. 2003;74(4):345–349. doi: 10.1016/S0367-326X(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 69.Zingare M. L., Zingare P. L., Dubey A. K., Ansari M. A. Clitoria ternatea (Aparajita): a review of the antioxidant, antidiabetic and hepatoprotective potentials. International Journal of Pharmacy and Biological Sciences. 2012;3(1):203–213. [Google Scholar]
- 70.Kamkaen N., Wilkinson J. M. The antioxidant activity of Clitoria ternatea flower petal extracts and eye gel. Phytotherapy Research. 2009;23(11):1624–1625. doi: 10.1002/ptr.2832. [DOI] [PubMed] [Google Scholar]
- 71.Taranalli A. D., Cheeramkuzhy T. C. Influence of Clitoria ternatea extracts on memory and central cholinergic activity in rats. Pharmaceutical Biology. 2000;38(1):51–56. doi: 10.1076/1388-0209(200001)3811-BFT051. [DOI] [PubMed] [Google Scholar]
- 72.Al-Howiriny T. A., Al-Sohaibani M. O., Al-Said M. S., Al-Yahya M. A., El-Tahir K. H., Rafatullah S. Hepatoprotective properties of Commiphora opobalsamum (“Balessan”), a traditional medicinal plant of Saudi Arabia. Drugs under Experimental and Clinical Research. 2004;30(5-6):213–220. [PubMed] [Google Scholar]
- 73.Dymock W., Hooper D., Warden C. J. H. Pharmacographia Indica. A History of the Principal Drugs of Vegetable Origin. London, UK: Trench, Trubner and Co.; 1890. [Google Scholar]
- 74.Al-Howiriny T. A., Al-Yahya M. A., Al-Said M. S., El-Tahir K. E. H., Rafatullah S. Studies on the pharmacological activities of an ethanol extract of balessan (Commiphora opobalsamum) Pakistan Journal of Biological Sciences. 2004;7(11):1933–1936. doi: 10.3923/pjbs.2004.1933.1936. [DOI] [Google Scholar]
- 75.Al-Howiriny T., Al-Sohaibani M., Al-Said M., Al-Yahya M., El-Tahir K., Rafatullah S. Effect of Commiphora opobalsamum (L.) Engl. (Balessan) on experimental gastric ulcers and secretion in rats. Journal of Ethnopharmacology. 2005;98(3):287–294. doi: 10.1016/j.jep.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 76.Anonymous . Unani Pharmacopoeia, vol. II. India, p. 21 (in Urdu) New Delhi, India: G.o.I. Unani Pharmacopoeial Committee; 2007. [Google Scholar]
- 77.Alter D. TURMERIC. http://www.herballegacy.com/Alter_History.html.
- 78.Yadav D., Yadav S. K., Khar R. K., Mujeeb M., Akhtar M. Turmeric (Curcuma longa L.): a promising spice for phytochemical and pharmacological activities. International Journal of Green Pharmacy. 2013;7(2):85–89. doi: 10.4103/0973-8258.116375. [DOI] [Google Scholar]
- 79.Ramirez-Bosca A., Soler A., Gutierrez M. A. C., Alvarez J. L., Almagro E. Q. Antioxidant curcuma extracts decrease the blood lipid peroxide levels of human subjects. Age. 1995;18(4):167–169. doi: 10.1007/BF02432631. [DOI] [Google Scholar]
- 80.Satoskar R. R., Shah S. J., Shenoy S. G. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. International Journal of Clinical Pharmacology Therapy and Toxicology. 1986;24(12):651–654. [PubMed] [Google Scholar]
- 81.Shankar T. N., Shantha N. V., Ramesh H. P., Murthy I. A., Murthy V. S. Toxicity studies on turmeric (Curcuma longa): acute toxicity studies in rats, guinea pigs and monkeys. Indian Journal of Experimental Biology. 1980;18(1):73–75. [PubMed] [Google Scholar]
- 82.Saad B., Said O. Greco-Arab and Islamic Herbal Medicine: Traditional System, Ethics, Safety, Efficacy, and Regulatory Issues. John Wiley & Sons; 2011. [Google Scholar]
- 83.Yaniv Z., Schafferman D., Amar Z. Tradition, uses and biodiversity of rocket (Eruca sativa, Brassicaceae) in Israel. Economic Botany. 1998;52(4):394–400. doi: 10.1007/BF02862069. [DOI] [Google Scholar]
- 84.Alqasoumi S. Carbon tetrachloride-induced hepatotoxicity: protective effect of “Rocket” Eruca sativa L. in rats. The American Journal of Chinese Medicine. 2010;38(1):75–88. doi: 10.1142/S0192415X10007671. [DOI] [PubMed] [Google Scholar]
- 85.Hussein J., Salah A., Oraby F., El-Deen A. N., El-Khayat Z. Antihepatotoxic effect of Eruca sativa extracts on alcohol induced liver injury in rats. Journal of American Science. 2010;6(11):381–389. [Google Scholar]
- 86.Lamy E., Schröder J., Paulus S., Brenk P., Stahl T., Mersch-Sundermann V. Antigenotoxic properties of Eruca sativa (rocket plant), erucin and erysolin in human hepatoma (HepG2) cells towards benzo(a)pyrene and their mode of action. Food and Chemical Toxicology. 2008;46(7):2415–2421. doi: 10.1016/j.fct.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 87.Sarwar Alam M., Kaur G., Jabbar Z., Javed K., Athar M. Eruca sativa seeds possess antioxidant activity and exert a protective effect on mercuric chloride induced renal toxicity. Food and Chemical Toxicology. 2007;45(6):910–920. doi: 10.1016/j.fct.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 88.Yehuda H., Khatib S., Sussan I., Musa R., Vaya J., Tamir S. Potential skin antiinflammatory effects of 4-methylthiobutylisothiocyanate (MTBI) isolated from rocket (Eruca sativa) seeds. BioFactors. 2009;35(3):295–305. doi: 10.1002/biof.32. [DOI] [PubMed] [Google Scholar]
- 89.Pérez C., Canal J. R., Campillo J. E., Romero A., Torres M. D. Hypotriglyceridaemic activity of Ficus carica leaves in experimental hypertriglyceridaemic rats. Phytotherapy Research. 1999;13(3):188–191. doi: 10.1002/(SICI)1099-1573(199905)13:3<188::AID-PTR411>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 90.Canal J. R., Torres M. D., Romero A., Pérez C. A chloroform extract obtained from a decoction of Ficus carica leaves improves the cholesterolaemic status of rats with streptozotocin-induced diabetes. Acta Physiologica Hungarica. 2000;87(1):71–76. doi: 10.1556/APhysiol.87.2000.1.8. [DOI] [PubMed] [Google Scholar]
- 91.Morton J. F. Fruits of Warm Climates. Winterville, NC, USA: Creative Resources Systems; 1987. [Google Scholar]
- 92.Aghel N., Kalantari H., Rezazadeh S. Hepatoprotective effect of Ficus carica leaf extract on mice intoxicated with carbon tetrachloride. Iranian Journal of Pharmaceutical Research. 2011;10(1):63–68. [PMC free article] [PubMed] [Google Scholar]
- 93.Singab A. N. B., Ayoub N. A., Ali E. N., Mostafa N. M. Antioxidant and hepatoprotective activities of Egyptian moraceous plants against carbon tetrachloride-induced oxidative stress and liver damage in rats. Pharmaceutical Biology. 2010;48(11):1255–1264. doi: 10.3109/13880201003730659. [DOI] [PubMed] [Google Scholar]
- 94.Mujeeb M., Khan S. A., Aeri V., Ali B. Hepatoprotective activity of the ethanolic extract of Ficus carica Linn. leaves in carbon tetrachloride-induced hepatotoxicity in rats. Iranian Journal of Pharmaceutical Research. 2011;10(2):301–306. [PMC free article] [PubMed] [Google Scholar]
- 95.Gond N., Khadabadi S. Hepatoprotective activity of Ficus carica leaf extract on rifampicin-induced hepatic damage in rats. Indian Journal of Pharmaceutical Sciences. 2008;70(3):364–366. doi: 10.4103/0250-474X.43003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ali B., Mujeeb M., Aeri V., Mir S. R., Faiyazuddin M., Shakeel F. Anti-inflammatory and antioxidant activity of Ficus carica Linn. leaves. Natural Product Research. 2012;26(5):460–465. doi: 10.1080/14786419.2010.488236. [DOI] [PubMed] [Google Scholar]
- 97.Joseph B., Justin Raj S. Pharmacognostic and phytochemical properties of Ficus carica Linn.—an overview. International Journal of PharmTech Research. 2011;3(1):8–12. [Google Scholar]
- 98.Bonamonte D., Foti C., Lionetti N., Rigano L., Angelini G. Photoallergic contact dermatitis to 8-methoxypsoralen in Ficus carica . Contact Dermatitis. 2010;62(6):343–348. doi: 10.1111/j.1600-0536.2010.01713.x. [DOI] [PubMed] [Google Scholar]
- 99.Al-Youssef H. M., Amina M., El-shafae A. M. Biological evaluation of constituents from Grewia mollis . Journal of Chemical and Pharmaceutical Research. 2012;4(1):508–518. [Google Scholar]
- 100.Louppe D., Oteng-Amoako A. A., Brink M. Plant Resources of Tropical Africa 7(1) Wageningen, The Netherlands: PROVA Foundation, Wageningen, The Netherlands; Backhuys Publishers, Lenden, The Netherlands; CTA; 2008. [Google Scholar]
- 101.Dalziel J. M. The Useful Plants of West Africa. London, UK: Crown Agents; 1937. [Google Scholar]
- 102.Asuku O., Atawodi S. E., Onyike E. Antioxidant, hepatoprotective, and ameliorative effects of methanolic extract of leaves of Grewia mollis Juss. on carbon tetrachloride-treated albino rats. Journal of Medicinal Food. 2012;15(1):83–88. doi: 10.1089/jmf.2010.0285. [DOI] [PubMed] [Google Scholar]
- 103.Obidah W., Godwin J. L., Fate J. Z., Madusolumuo M. A. Toxic effects of Grewia mollis stem bark in experimental rats. Journal of American Science. 2010;6(12):1544–1548. [Google Scholar]
- 104.Safa O., Soltanipoor M. A., Rastegar S., Kazemi M., Nourbakhsh Dehkordi K., Ghannadi A. An ethnobotanical survey on Hormozgan province, Iran. Avicenna Journal of Phytomedicine. 2012;3(1):64–81. [PMC free article] [PubMed] [Google Scholar]
- 105.Al-Said M. S., Mothana R. A., Al-Sohaibani M. O., Rafatullah S. Ameliorative effect of Grewia tenax (Forssk) fiori fruit extract on CCl4-induced oxidative stress and hepatotoxicity in rats. Journal of Food Science. 2011;76(9):T200–T206. doi: 10.1111/j.1750-3841.2011.02381.x. [DOI] [PubMed] [Google Scholar]
- 106.Saleem N. H., Ferro V. A., Simpson A. M., Igoli J., Gray A. I., Drummond R. M. The inhibitory effect of Haloxylon salicornicum on contraction of the mouse uterus. Evidence-Based Complementary and Alternative Medicine. 2013;2013 doi: 10.1155/2013/714075.714075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shafi M. S., Ashraf M. Y., Sarwar G. Wild medicinal plants of Cholistan area of Pakistan. Pakistan Journal of Biological Sciences. 2001;4(1):112–116. doi: 10.3923/pjbs.2001.112.116. [DOI] [Google Scholar]
- 108.Wariss H. M., Ahmad S., Anjum S., Alam K. Ethnobotanical studies of dicotyledonous plants of Lal Suhanra National Park, Bahawalpur, Pakistan. International Journal of Science and Research. 2012;3(6):2452–2460. [Google Scholar]
- 109.Ahmad M., Eram S. Hepatoprotective studies on Haloxylon salicornicum: a plant from Cholistan desert. Pakistan Journal of Pharmaceutical Sciences. 2011;24(3):377–382. [PubMed] [Google Scholar]
- 110.Alqasoumi S. I., Soliman G. A. E. H., Awaad A. S., Donia A. E. R. M. Anti-inflammatory activity, safety and protective effects of Leptadenia pyrotechnica, Haloxylon salicornicum and Ochradenus baccatus in ulcerative colitis. Phytopharmacology. 2012;2(1):58–71. [Google Scholar]
- 111.Ferheen S., Ahmed E., Afza N., Malik A. Phytochemical studies on Haloxylon salicornicum . Journal of the Chemical Society of Pakistan. 2005;27(2):219–222. [Google Scholar]
- 112.Roman I., Cristescu M., Puica C. Effects of Hypericum perforatum and Hypericum maculatum extracts administration on some morphological and biochemical parameters in rat liver intoxicated with alcohol. Studia Universitatis “Vasile Goldiş”, Seria Ştiinţele Vieţii. 2011;21(2):361–370. [Google Scholar]
- 113.Ozturk Y., Aydin S., Baser K. H. C., Kirimer N., Kurtar-Ozturk N. Hepatoprotective activity of Hypericum perforatum L. alcoholic extract in rodents. Phytotherapy Research. 1992;6(1):44–46. doi: 10.1002/ptr.2650060111. [DOI] [Google Scholar]
- 114.Celen C., Ozkan S., Ayhan F. The phenolic compounds from Hypericum perforatum and their antimicrobial activities. Hacettepe Journal of Biology and Chemistry. 2008;36(4):339–345. [Google Scholar]
- 115.Greeson J. M., Sanford B., Monti D. A. St. John's wort (Hypericum perforatum): a review of the current pharmacological, toxicological, and clinical literature. Psychopharmacology. 2001;153(4):402–414. doi: 10.1007/s002130000625. [DOI] [PubMed] [Google Scholar]
- 116.Alqasoumi S. I. Isolation and Chemical Structure Elucidation of Hepatoprotective Constituents from Plants Used in Traditional Medicine in Saudi Arabia. College of Pharmacy, King Saud University; 2007. [Google Scholar]
- 117.Alqasoumi S. I., Abdel-Kader M. S. Terpenoids from Juniperus procera with hepatoprotective activity. Pakistan Journal of Pharmaceutical Sciences. 2012;25(2):315–322. [PubMed] [Google Scholar]
- 118.Manohar D., Viswanatha G. L., Nagesh S., Jain V., Shivaprasad H. N. Ethnopharmacology of Lepidium sativum Linn. (Brassicaceae): a review. International Journal of Phytothearpy Research. 2012;2(1):1–7. [Google Scholar]
- 119.Biswas S. K., Chowdhury A., Das J., Roy A., Hosen S. M. Z. Pharmacological potentials of Moringa oleifera Lam.: a review. International Journal of Pharmaceutical Science Research. 2012;3(2):305–310. [Google Scholar]
- 120.Mahajan S. G., Mehta A. A. Effect of Moringa oleifera Lam. seed extract on ovalbumin-induced airway inflammation in guinea pigs. Inhalation Toxicology. 2008;20(10):897–909. doi: 10.1080/08958370802027443. [DOI] [PubMed] [Google Scholar]
- 121.Mazu̧mder U. K., Gupta M., Chakrabarti S., Pal D. Evaluation of hematological and hepatorenal functions of methanolic extract of Moringa oleifera Lam. root treated mice. Indian Journal of Experimental Biology. 1999;37(6):612–614. [PubMed] [Google Scholar]
- 122.Anwar F., Latif S., Ashraf M., Gilani A. H. Moringa oleifera: a food plant with multiple medicinal uses. Phytotherapy Research. 2007;21(1):17–25. doi: 10.1002/ptr.2023. [DOI] [PubMed] [Google Scholar]
- 123.Tariq M. Nigella sativa seeds: folklore treatment in modern day medicine. Saudi Journal of Gastroenterology. 2008;14(3):105–106. doi: 10.4103/1319-3767.41725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Khan M. L. A. Tibb-Al-Nabvi: Nigella sativa . Islamic Voice. 1999;13–18(152):1–2. [Google Scholar]
- 125.Ahmad A., Husain A., Mujeeb M., Khan S. A., Najmi A. K., Siddique N. A., Damanhouri Z. A., Anwar F. A review on therapeutic potential of Nigella sativa: a miracle herb. Asian Pacific Journal of Tropical Biomedicine. 2013;3(5):337–352. doi: 10.1016/S2221-1691(13)60075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sharma A., Sharma M. S., Mishra A., Sharma S., Kumar B., Bhandari A. A review on the plants used in liver disease. International Journal of Research in Pharmacy and Chemistry. 2011;1(2):224–236. [Google Scholar]
- 127.Diwan S. Y. Effect of Peganum harmala methanol extract on liver and kidney of mice administered MTX drug. Journal of Al-Nahrain University. 2013;16(4):161–166. [Google Scholar]
- 128.Gulshan A. B., Dasti A. A., Hussain S., Atta M. I., Amin-ud-Din M. Indigenous uses of medicinal plants in rural areas of Dera Ghazi Khan, Punjab, Pakistan. Journal of Agricultural and Biological Science. 2012;7(9):750–762. [Google Scholar]
- 129.Ratsch C. The Encyclopedia of Psychoactive Plants: Ethnopharmacology and Its Application. Rochester, Vt, USA: Park Street Press; 1998. [Google Scholar]
- 130.Asgarpanah J., Ramezanloo F. Chemistry, pharmacology and medicinal properties of Peganum harmala L. African Journal of Pharmacy and Pharmacology. 2012;6(22):1573–1580. [Google Scholar]
- 131.Hamden K., Masmoudi H., Ellouz F., Elfeki A., Carreau S. Protective effects of Peganum harmala extracts on thiourea-induced diseases in adult male rat. Journal of Environmental Biology. 2008;29(1):73–77. [PubMed] [Google Scholar]
- 132.Bhaskar V. H., Balakrishnan N. Analgesic, anti-inflammatory and antipyretic activities of Pergularia daemia and Carissa carandas . Daru. 2009;17(3):168–174. [Google Scholar]
- 133.Bhaskar V. H., Balakrishnan N. Protective effects of Pergularia daemia roots against paracetamol and carbon tetrachloride-induced hepatotoxicity in rats. Pharmaceutical Biology. 2010;48(11):1265–1272. doi: 10.3109/13880201003730667. [DOI] [PubMed] [Google Scholar]
- 134.Sureshkumar S. V., Mishra S. H. Hepatoprotective effect of extracts from Pergularia daemia Forsk. Journal of Ethnopharmacology. 2006;107(2):164–168. doi: 10.1016/j.jep.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 135.Kumar S. V. S., Mishra S. H. Hepatoprotective effect of Pergularia daemia (Forsk.) ethanol extract and its fraction. Indian Journal of Experimental Biology. 2008;46(6):447–452. [PubMed] [Google Scholar]
- 136.Hoffmann D. Medical Herbalism: The Science and Practice of Herbal Medicine. Healing Arts Press; 2010. [Google Scholar]
- 137.Leung Y. A. Encyclopedia of Common Natural Ingredients Used in Food, Drug and Cosmetics. New York, NY, USA: John Wiley & Sons; 1988. [Google Scholar]
- 138.Al-Howiriny T. A., Al-Sohaibani M. O., El-Tahir K. H., Rafatullah S. Preliminary evaluation of the anti-inflammatory and anti-hepatotoxic activities of ‘Parsley’ Petroselinum crispum in rats. Journal of Natural Remedies. 2003;3(1):54–62. [Google Scholar]
- 139.Eckey-Kaltenbach H., Ernst D., Heller W., Sandermann Jnr H. Biochemical plant responses to ozone. IV.Cross-induction of defensive pathways in parsley (Petroselinum crispum L.) plants. Plant Physiology. 1994;104(1):67–74. doi: 10.1104/pp.104.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chaves D. S. A., Frattani F. S., Assafim M., de Almeida A. P., Zingali R. B., Costa S. S. Phenolic chemical composition of Petroselinum crispum extract and its effect on haemostasis. Natural Product Communications. 2011;6(7):961–964. [PubMed] [Google Scholar]
- 141.Calixto J. O. B., Santos A. R. S., Yunes R. A. A review of the plants of the genus Phyllanthus: their chemistry, pharmacology, and therapeutic potential. Medicinal Research Reviews. 1998;18(4):225–258. doi: 10.1002/(sici)1098-1128(199807)18:4<225::aid-med2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 142.Asha V. V., Sheeba M. S., Suresh V., Wills P. J. Hepatoprotection of Phyllanthus maderaspatensis against experimentally induced liver injury in rats. Fitoterapia. 2007;78(2):134–141. doi: 10.1016/j.fitote.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 143.Karthikeyan M., Mathews J. A., Annamalai A. Antibacterial activity of Phyllanthus maderaspatensis leaves extract. Pharma Science Monitor. 2012;3(3):1–10. [Google Scholar]
- 144.Ravichandran N., Vajrai R., Raj C. D., Brindha P. Phytochemical analysis and in vitro cytotoxic effect of Phyllanthus madraspatensis L. International Journal of Pharmacy and Pharmaceutical Sciences. 2012;4(2):111–114. [Google Scholar]
- 145.Jonas W. B. Mosby's Dictionary of Complementary and Alternative Medicine. Mosby; 2005. [Google Scholar]
- 146.Özcan M. M., Chalchat J. C. Chemical composition and antifungal effect of anise (Pimpinella anisum L.) fruit oil at ripening stage. Annals of Microbiology. 2006;56(4):353–358. doi: 10.1007/BF03175031. [DOI] [Google Scholar]
- 147.Shojaii A., Fard M. A. Review of pharmacological properties and chemical constituents of Pimpinella anisum . ISRN Pharmaceutics. 2012;2012 doi: 10.5402/2012/510795.510795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ahmad M., Itoo A., Baba I., Jain S. M., Saxena R. C. Hepatoprotective activity of Portulaca oleracea Linn. on experimental animal model. International Journal of Pharmacy and Pharmaceutical Sciences. 2013;5(3):267–269. [Google Scholar]
- 149.Anusha M., Venkateswarlu M., Prabhakaran V., Taj S. S., Kumari B. P., Ranganayakulu D. Hepatoprotective activity of aqueous extract of Portulaca oleracea in combination with lycopene in rats. Indian Journal of Pharmacology. 2011;43(5):563–567. doi: 10.4103/0253-7613.84973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rasheed A. N., Afifi F. U., Shaedah M., Taha M. O. Investigation of the active constituents of Portulaca oleraceae L. (Portulacaceae) growing in Jordan. Pakistan Journal of Pharmaceutical Sciences. 2004;17(1):37–45. [PubMed] [Google Scholar]
- 151.Chowdhary C. V., Meruva A., Naresh K., Elumalai R. K. A. A review on phytochemical and pharmacological profile of Portulaca oleracea Linn. (Purslane) International Journal of Research in Ayurveda and Pharmacy. 2013;4(1):34–37. doi: 10.7897/2277-4343.04119. [DOI] [Google Scholar]
- 152.Deshwal N., Sharma A. K., Sharma P. Review on hepatoprotective plants. International Journal of Pharmaceutical Sciences Review and Research. 2011;7(1):15–26. [Google Scholar]
- 153.Al Gonemi A. A. Encyclopaedia of the United Arab Emirates Plants Used in Folk Medicine. Al-Ain, UAE: United Arab Emirates University Press; 1992. (Arabic) [Google Scholar]
- 154.Marwat S. K., Rehman F., Usman K., Shah S. S., Anwar N., Ullah I. A review of phytochemistry, bioactivities and ethno medicinal uses of Rhazya stricta Decsne (Apocynaceae) African Journal of Microbiology Research. 2012;6(8):1629–1641. [Google Scholar]
- 155.Ali B. H., Al-Qarawi A. A., Bashir A. K., Tanira M. O. Phytochemistry, pharmacology and toxicity of Rhazya stricta decne: a review. Phytotherapy Research. 2000;14(4):229–234. doi: 10.1002/1099-1573(200006)14:4<229::aid-ptr673>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 156.Ghani M. A. Khazeenat-Al-Advia. Vol. 111. Lucknow, India: Matbaa Naval Kishore; 1921. [Google Scholar]
- 157.Raza A. K. Tazkiratul-Hind, Yadgaar Razaee. Hyderabad, India: Shamsul-Islam Press; 1933. [Google Scholar]
- 158.Albert F. H. Economic Botany. New York, NY, USA: McGraw-Hill; 1952. [Google Scholar]
- 159.Ageel A. M., Mossa J. S., Al-Yahya M. A., Al-Said M. S., Tariq M. Experimental studies on antirheumatic crude drugs used in Saudi traditional medicine. Drugs under Experimental and Clinical Research. 1989;15(8):369–372. [PubMed] [Google Scholar]
- 160.Anonymous Tropical plant database. http://www.rain-tree.com/sarsaparilla.htm#.VAsc-Pm1agw.
- 161.Dash B. Herbal Cure: Jaundice and Liver Disorders. B. Jain Publishers; 2004. [Google Scholar]
- 162.Sultana S., Perwaiz S., Iqbal M., Athar M. Crude extracts of hepatoprotective plants, Solanum nigrum and Cichoriunz intybus inhibit free radical-mediated DNA damage. Journal of Ethnopharmacology. 1995;45(3):189–192. doi: 10.1016/0378-8741(94)01214-K. [DOI] [PubMed] [Google Scholar]
- 163.Wannang N. N., Anuka J. A., Kwanashie H. O., Gyang S. S., Auta A. Anti-seizure activity of the aqueous leaf extract of Solanum nigrum Linn. (solanaceae) in experimental animals. African Health Sciences. 2008;8(2):74–79. [PMC free article] [PubMed] [Google Scholar]
- 164.Jain R., Sharma A., Gupta S., Sarethy I. P., Gabrani R. Solanum nigrum: current perspectives on therapeutic properties. Alternative Medicine Review. 2011;16(1):78–85. [PubMed] [Google Scholar]
- 165.Lin H.-M., Tseng H.-C., Wang C.-J., Lin J.-J., Lo C.-W., Chou F.-P. Hepatoprotective effects of Solanum nigrum Linn. extract against CCl4-iduced oxidative damage in rats. Chemico-Biological Interactions. 2008;171(3):283–293. doi: 10.1016/j.cbi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 166.Singh S., Sharma S. K., Mann R. Pharmacognostical standardization of stem of Suaeda maritima (L.) Dumort. International Journal of Pharmacy and Pharmaceutical Sciences. 2012;4(3):304–306. [Google Scholar]
- 167.Ravikumar S., Gnanadesigan M., Jacob Inbaneson S., Kalaiarasi A. Hepatoprotective and antioxidant properties of Suaeda maritima (L.) Dumort ethanolic extract on concanavalin-A induced hepatotoxicity in rats. Indian Journal of Experimental Biology. 2011;49(6):455–460. [PubMed] [Google Scholar]
- 168.Singh S., Mann R., Sharma S. K. Pharmacognostical standardization of root of Suaeda maritima (L.) dumort. Der Pharmacia Lettre. 2013;5(1):116–120. [Google Scholar]
- 169.Sina I. Al Qanoon Fil Tib. Vol. 522. Lucknow, India: Mataba Munshi Naval Kishore; 2007. [Google Scholar]
- 170.Bakr R. O., El Raey M. A. E.-A., Ashour R. S. Phenolic content, radical scavenging activity and cytotoxicity of Tamarix nilotica (Ehrenb.) bunge growing in Egypt. Journal of Pharmacognosy and Phytotherapy. 2013;5(3):47–52. doi: 10.5897/JPP12.062. [DOI] [Google Scholar]
- 171.Abouzid S., Sleem A. Hepatoprotective and antioxidant activities of Tamarix nilotica flowers. Pharmaceutical Biology. 2011;49(4):392–395. doi: 10.3109/13880209.2010.518971. [DOI] [PubMed] [Google Scholar]
- 172.Sree R. M., Srinivasan M. Hepatoprotective effect of Tephrosia purprea in experimental animals. Indian Journal of Pharmacology. 1993;25(1):34–36. [Google Scholar]
- 173.Kumar A., Dutta M., Bhatt T. K., Dalal D. S. Use of herbal liver tonic yakrifit in equine practice. Indian Veterinary Journal. 1997;74(5):424–425. [Google Scholar]
- 174.Jitendra P. A., Pravin T. A. Prospective use of Tephrosia purpurea in remedial treatment of PCOS: study in Wistar rat. ISCA Journal of Biological Sciences. 2012;1(3):1–6. [Google Scholar]
- 175.Khatri A., Garg A., Agrawal S. S. Evaluation of hepatoprotective activity of aerial parts of Tephrosia purpurea L. and stem bark of Tecomella undulata . Journal of Ethnopharmacology. 2009;122(1):1–5. doi: 10.1016/j.jep.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 176.Janbaz K. H., Qadir M. I., Jan A., Gilani A. H. Anti-diarrheal activity of methanolic extract of Tephrosia purpurea . Acta Poloniae Pharmaceutica—Drug Research. 2013;70(2):345–347. [PubMed] [Google Scholar]
- 177.Tariq M., Ageel A. M., Al-Yahya M. A., Mossa J. S., Al-Said M. S. Anti-inflammatory activity of Teucrium polium . International Journal of Tissue Reactions. 1989;11(4):185–188. [PubMed] [Google Scholar]
- 178.Panovska T. K., Kulevanova S., Gjorgoski I., Bogdanova M., Petrushevska G. Hepatoprotective effect of the ethyl acetate extract of Teucrium polium L. against carbontetrachloride-induced hepatic injury in rats. Acta Pharmaceutica. 2007;57(2):241–248. doi: 10.2478/v10007-007-0020-x. [DOI] [PubMed] [Google Scholar]
- 179.Gholivand M. B., Piryaei M., Abolghasemi M. M., Maassoumi S. M. Rapid analysis of volatile components from Teucrium polium L. by nanoporous silica-polyaniline solid phase microextraction fibre. Phytochemical Analysis. 2013;24(1):69–74. doi: 10.1002/pca.2382. [DOI] [PubMed] [Google Scholar]
- 180.Bahramikia S., Yazdanparast R. Phytochemistry and medicinal properties of Teucrium polium L. (Lamiaceae) Phytotherapy Research. 2012;26(11):1581–1593. doi: 10.1002/ptr.4617. [DOI] [PubMed] [Google Scholar]
- 181.Moustapha C., Taher Hasen M. W., Sadaka M. Chemical constituents of Teucrium polium L. var. mollissimum Hand-Mazz. Jordan Journal of Chemistry. 1975;6(3):339–345. [Google Scholar]
- 182.Shivhare M. K., Singour P. K., Chaurasiya P. K., Pawar R. S. Trianthema portulacastrum Linn. (bishkhapra) Pharmacognosy Reviews. 2012;6(12):132–140. doi: 10.4103/0973-7847.99947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Banu S. G., Kumar G., Murugesan A. G. Ethanolic leaves extract of Trianthema portulacastrum L. ameliorates aflatoxin B1 induced hepatic damage in rats. Indian Journal of Clinical Biochemistry. 2009;24(3):250–256. doi: 10.1007/s12291-009-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Amin A., Lotfy M., Shafiullah M., Adeghate E. The protective effect of Tribulus terrestris in diabetes. Annals of the New York Academy of Sciences. 2006;1084:391–401. doi: 10.1196/annals.1372.005. [DOI] [PubMed] [Google Scholar]
- 185.Kostova I., Dinchev D. Saponins in Tribulus terrestris—chemistry and bioactivity. Phytochemistry Reviews. 2005;4(2-3):111–137. doi: 10.1007/s11101-005-2833-x. [DOI] [Google Scholar]
- 186.Aboagarib E. A. A., Yang R., Hua X., Siddeeg A. Chemical compositions, nutritional properties and volatile compounds of guddaim (Grewia tenax. Forssk) Fiori Fruits. Journal of Food and Nutrition Research. 2014;2(4):187–192. [Google Scholar]
- 187.Al-Shanawani M. A. Plant Used in Saudi Folk Medicine. Riyadh, Saudi Arabia: King Abdul-Aziz City for Science and Technology; 1996. [Google Scholar]
- 188.El-Shazly A. M., Dora G., Wink M. Alkaloids of Haloxylon salicornicum (Moq.) Bunge ex Boiss. (Chenopodiaceae) Pharmazie. 2005;60(12):949–952. [PubMed] [Google Scholar]
- 189.Istikoglou C. I., Mavreas V., Geroulanos G. History and therapeutic properties of Hypericum perforatum from antiquity until today. Psychiatrikē. 2010;21(4):332–338. [PubMed] [Google Scholar]
- 190.Aliasl J., Khoshzaban F. Traditional herbal remedies for burn wound healing in canon of Avicenna. Jundishapur Journal of Natural Pharmaceutical Products. 2013;8(4):192–196. [Google Scholar]
- 191.Šavikin K., Dobrić S., Tadić V., Zdunić G. Antiinflammatory activity of ethanol extracts of Hypericum perforatum L., H. barbatum Jacq., H. hirsutum L., H. richeri Vill. and H. androsaemum L. in rats. Phytotherapy Research. 2007;21(2):176–180. doi: 10.1002/ptr.2041. [DOI] [PubMed] [Google Scholar]
- 192.Kumar V., Singh P. N., Bhattacharya S. K. Anti-inflammatory and analgesic activity of Indian Hypericum perforatum L. Indian Journal of Experimental Biology. 2001;39(4):339–343. [PubMed] [Google Scholar]
- 193.Andersen F. A. Final report on the safety assessment of Hypericum perforatum extract and Hypericum perforatum oil. International Journal of Toxicology. 2001;20(supplement 2):31–39. doi: 10.1080/10915810160233749. [DOI] [PubMed] [Google Scholar]
- 194.Burits M., Asres K., Bucar F. The antioxidant activity of the essential oils of Artemisia afra, Artemisia abyssinica and Juniperus procera . Phytotherapy Research. 2001;15(2):103–108. doi: 10.1002/ptr.691. [DOI] [PubMed] [Google Scholar]
- 195.Tunón H., Olavsdotter C., Bohlin L. Evaluation of anti-inflammatory activity of some Swedish medicinal plants. Inhibition of prostaglandin biosynthesis and PAF-induced exocytosis. Journal of Ethnopharmacology. 1995;48(2):61–76. doi: 10.1016/0378-8741(95)01285-L. [DOI] [PubMed] [Google Scholar]
- 196.Abuelgasim A. I., Nuha H. S., Mohammed A. H. Hepatoprotective effect of Lepidium sativum against carbon tetrachloride induced damage in rats. Research Journal of Animal & Veterinary Sciences. 2008;3:20–23. [Google Scholar]
- 197.Yadav Y. C., Srivastav D. N., Seth A. K., Saini V., Balaraman R., Ghelani T. K. In vivo antioxidant potential of Lepidium sativum L. seeds in albino rats using cisplatin induced nephrotoxicity. International Journal of Phytomedicine. 2010;2(3):292–298. doi: 10.5138/ijpm.2010.0975.0185.02041. [DOI] [Google Scholar]
- 198.Zia-Ul-Haq M., Ahmad S., Calani L., Mazzeo T., Del Rio D., Pellegrini N., de Feo V. Compositional study and antioxidant potential of Ipomoea hederacea Jacq. and Lepidium sativum L. seeds. Molecules. 2012;17(9):10306–10321. doi: 10.3390/molecules170910306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Agarwal J., Verma D. L. Antioxidant activity-guided fractionation of aqueous extracts from Lepidium sativum and identification of active flavonol glycosides. Academia Arena. 2011;3(12):14–18. [Google Scholar]
- 200.Raval N. D., Ravishankar B., Ashok B. K. Anti-inflammatory effect of Chandrashura (Lepidium sativum Linn.) an experimental study. Ayu. 2013;34(3):302–304. doi: 10.4103/0974-8520.123132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Adam S. E. I. Effects of various levels of dietary Lepidium sativum L. seeds in rats. The American Journal of Chinese Medicine. 1999;27(3-4):397–405. doi: 10.1142/S0192415X99000458. [DOI] [PubMed] [Google Scholar]
- 202.Ajilore B. S., Atere T. G., Oluogun W. A., Aderemi V. A. Protective effects of Moringa oleifera Lam. on cadmium-induced liver and kidney damage in male Wistar rats. International Journal of Phytotherapy Research. 2012;2(3):42–50. [Google Scholar]
- 203.Pari L., Kumar N. A. Hepatoprotective activity of Moringa oleifera on antitubercular drug-induced liver damage in rats. Journal of Medicinal Food. 2002;5(3):171–177. doi: 10.1089/10966200260398206. [DOI] [PubMed] [Google Scholar]
- 204.Sreelatha S., Padma P. R. Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods for Human Nutrition. 2009;64(4):303–311. doi: 10.1007/s11130-009-0141-0. [DOI] [PubMed] [Google Scholar]
- 205.Minaiyan M., Asghari G., Taheri D., Saeidi M., Nasr-Esfahani S. Anti-inflammatory effect of Moringa oleifera Lam. seeds on acetic acid-induced acute colitis in rats. Avicenna Journal of Phytomedicine. 2014;4(2):127–136. [PMC free article] [PubMed] [Google Scholar]
- 206.Rathi B. S., Bodhankar S. L., Baheti A. M. Evaluation of aqueous leaves extract of Moringa oleifera Linn. for wound healing in albino rats. Indian Journal of Experimental Biology. 2006;44(11):898–901. [PubMed] [Google Scholar]
- 207.Yildiz F., Coban S., Terzi A., Ates M., Aksoy N., Cakir H., Ocak A. R., Bitiren M. Nigella sativa relieves the deleterious effects of ischemia reperfusion injury on liver. World Journal of Gastroenterology. 2008;14(33):5204–5209. doi: 10.3748/wjg.14.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Al-Suhaimi E. A. Hepatoprotective and immunological functions of Nigella sativa seed oil against hypervitaminosis A in adult male rats. International Journal for Vitamin and Nutrition Research. 2012;82(4):288–297. doi: 10.1024/0300-9831/a000121. [DOI] [PubMed] [Google Scholar]
- 209.Gani A. M. S., John S. A. Evalution of hepatoprotective effect of Nigella sativa L. International Journal of Pharmacy and Pharmaceutical Sciences. 2013;5(4):428–430. [Google Scholar]
- 210.Al-Ghamdi M. S. Protective effect of Nigella sativa seeds against carbon tetrachloride-induced liver damage. American Journal of Chinese Medicine. 2003;31(5):721–728. doi: 10.1142/S0192415X03001399. [DOI] [PubMed] [Google Scholar]
- 211.Danladi J., Abdulsalam A., Timbuak J. A., Ahmed S. A., Aa M., Dahiru A. U. Hepatoprotective effect of black seed (Nigella sativa) oil on carbon tetrachloride (CCl4) induced liver toxicity in adult wistar rats. IOSR-Journal of Dental and Medical Science. 2013;4(3):56–62. doi: 10.9790/0853-0435662. [DOI] [Google Scholar]
- 212.Alemi M., Sabouni F., Sanjarian F., Haghbeen K., Ansari S. Anti-inflammatory effect of seeds and Callus of Nigella sativa L. extracts on mix glial cells with regard to their thymoquinone content. AAPS PharmSciTech. 2013;14(1):160–167. doi: 10.1208/s12249-012-9899-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Chehl N., Chipitsyna G., Gong Q., Yeo C. J., Arafat H. A. Anti-inflammatory effects of the Nigella sativa seed extract, thymoquinone, in pancreatic cancer cells. HPB. 2009;11(5):373–381. doi: 10.1111/j.1477-2574.2009.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Al-Ghamdi M. S. The anti-inflammatory, analgesic and antipyretic activity of Nigella sativa . Journal of Ethnopharmacology. 2001;76(1):45–48. doi: 10.1016/S0378-8741(01)00216-1. [DOI] [PubMed] [Google Scholar]
- 215.Boskabady M.-H., Keyhanmanesh R., Khameneh S., Doostdar Y., Khakzad M.-R. Potential immunomodulation effect of the extract of Nigella sativa on ovalbumin sensitized guinea pigs. Journal of Zhejiang University: Science B. 2011;12(3):201–209. doi: 10.1631/jzus.B1000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Leong X.-F., Mustafa M. R., Jaarin K. Nigella sativa and its protective role in oxidative stress and hypertension. Evidence-Based Complementary and Alternative Medicine. 2013;2013 doi: 10.1155/2013/120732.120732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zaoui A., Cherrah Y., Mahassini N., Alaoui K., Amarouch H., Hassar M. Acute and chronic toxicity of Nigella sativa fixed oil. Phytomedicine. 2002;9(1):69–74. doi: 10.1078/0944-7113-00084. [DOI] [PubMed] [Google Scholar]
- 218.Tanweer A. J., Chand N., Khan S., et al. Association of Peganum harmala L. supplementation with liver function test of broiler chicks. Pakistan Journal of Science. 2012;64(1):75–79. [Google Scholar]
- 219.Ahmed H., Abu El Zahab H., Alswiai G. Purification of antioxidant protein isolated from Peganum harmala and its protective effect against CCl4 toxicity in rats. Turkish Journal of Biology. 2013;37(1):39–48. doi: 10.3906/biy-1110-29. [DOI] [Google Scholar]
- 220.Bremner P., Rivera D., Calzado M. A., Obón C., Inocencio C., Beckwith C., Fiebich B. L., Muñoz E., Heinrich M. Assessing medicinal plants from South-Eastern Spain for potential anti-inflammatory effects targeting nuclear factor-Kappa B and other pro-inflammatory mediators. Journal of Ethnopharmacology. 2009;124(2):295–305. doi: 10.1016/j.jep.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 221.Muhi-Eldeen Z., Al-Shamma K. J., Al-Hussainy T. M., Al-Kaissi E. N., Al-Daraji A. M., Ibrahim H. Acute toxicological studies on the extract of Iraqi Peganum harmala in rats. European Journal of Scientific Research. 2008;22(4):494–500. [Google Scholar]
- 222.Lamchouri F., Settaf A., Cherrah Y., El Hamidi M., Tligui N. S., Lyoussi B., Hassar M. Experimental toxicity of Peganum harmala seeds. Annales Pharmaceutiques Francaises. 2002;60(2):123–129. [PubMed] [Google Scholar]
- 223.Herraiz T., González D., Ancín-Azpilicueta C., Arán V. J., Guillén H. β-carboline alkaloids in Peganum harmala and inhibition of human monoamine oxidase (MAO) Food and Chemical Toxicology. 2010;48(3):839–845. doi: 10.1016/j.fct.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 224.Sravani K. M. R., Kumar C. K. A., Lakshmi S. M., Rani G. S. Psychopharmacological profiles of Pergularia daemia (forsk.) Chiov. Asian Journal of Pharmaceutical and Clinical Research. 2012;5(2):112–114. [Google Scholar]
- 225.Zhang H., Chen F., Wang X. I., Yao H.-Y. Evaluation of antioxidant activity of parsley (Petroselinum crispum) essential oil and identification of its antioxidant constituents. Food Research International. 2006;39(8):833–839. doi: 10.1016/j.foodres.2006.03.007. [DOI] [Google Scholar]
- 226.Wong P. Y. Y., Kitts D. D. Studies on the dual antioxidant and antibacterial properties of parsley (Petroselinum crispum) and cilantro (Coriandrum sativum) extracts. Food Chemistry. 2006;97(3):505–515. doi: 10.1016/j.foodchem.2005.05.031. [DOI] [Google Scholar]
- 227.Bown D. Encyclopedia of Herbs and Their Uses. London, UK: Dorling Kindersley; 1995. [Google Scholar]
- 228.Munshi A., Mehrotra R., Panda S. K. Evaluation of Phyllanthus amarus and Phyllanthus maderaspatensis as agents for postexposure prophylaxis in neonatal duck hepatitis B virus infection. Journal of Medical Virology. 1993;40(1):53–58. doi: 10.1002/jmv.1890400111. [DOI] [PubMed] [Google Scholar]
- 229.Kumaran A., Karunakaran R. J. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT: Food Science and Technology. 2007;40(2):344–352. doi: 10.1016/j.lwt.2005.09.011. [DOI] [Google Scholar]
- 230.Rao Y. K., Fang S.-H., Tzeng Y.-M. Anti-inflammatory activities of constituents isolated from Phyllanthus polyphyllus . Journal of Ethnopharmacology. 2006;103(2):181–186. doi: 10.1016/j.jep.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 231.Kuttan R., Harikumar K. B. Phyllanthus Species: Scientific Evaluation and Medicinal Applications. New York, NY, USA: CRC Press; 2011. [Google Scholar]
- 232.Cengiz N., Ozbek H., Him A. Hepatoprotective effects of Pimpinella anisum seed extract in rats. Pharmacologyonline. 2008;3:870–874. [Google Scholar]
- 233.Al-Ismail K. M., Aburjai T. Antioxidant activity of water and alcohol extracts of chamomile flowers, anise seeds and dill seeds. Journal of the Science of Food and Agriculture. 2004;84(2):173–178. doi: 10.1002/jsfa.1625. [DOI] [Google Scholar]
- 234.Gülçin I., Oktay M., Kireçci E., Küfrevıoǧlu Ö. I. Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) seed extracts. Food Chemistry. 2003;83(3):371–382. doi: 10.1016/S0308-8146(03)00098-0. [DOI] [Google Scholar]
- 235.Tas A., Özbek H., Atasoy N., Altug M. E., Ceylan E. Evaluation of analgesic and antiinflammatory activity of Pimpinella anisum fixed oil extract. Indian Veterinary Journal. 2006;83(8):840–843. [Google Scholar]
- 236.Gosselin R. E., Smith R. P., Hodge H. C. Clinical Toxicology of Commercial Product. 5th. Vol. 2. Baltimore, Md, USA: Williams & Wilkins; 1984. [Google Scholar]
- 237.Hanefi O., Mustafa O., Abdurrahman O., Ebubekir C., Zabit Y. Determination of lethal doses of volatile and fixed oils of several plants. Eastern Journal of Medicine. 2002;9(1):4–6. [Google Scholar]
- 238.Mohamed A. I., Hussein A. S. Chemical composition of purslane (Portulaca oleracea) Plant Foods for Human Nutrition. 1994;45(1):1–9. doi: 10.1007/BF01091224. [DOI] [PubMed] [Google Scholar]
- 239.Elkhayat E. S., Ibrahim S. R. M., Aziz M. A. Portulene, a new diterpene from Portulaca oleracea L. Journal of Asian Natural Products Research. 2008;10(11):1039–1043. doi: 10.1080/10286020802320590. [DOI] [PubMed] [Google Scholar]
- 240.Lim Y. Y., Quah E. P. L. Antioxidant properties of different cultivars of Portulaca oleracea . Food Chemistry. 2007;103(3):734–740. doi: 10.1016/j.foodchem.2006.09.025. [DOI] [Google Scholar]
- 241.Chan K., Islam M. W., Kamil M., Radhakrishnan R., Zakaria M. N. M., Habibullah M., Attas A. The analgesic and anti-inflammatory effects of Portulaca oleracea L. subsp. sativa (Haw.) Celak. Journal of Ethnopharmacology. 2000;73(3):445–451. doi: 10.1016/S0378-8741(00)00318-4. [DOI] [PubMed] [Google Scholar]
- 242.Musa K. Y., Ahmed A., Ibrahim G., Ojonugwa O. E., Bisalla M., Musa H., Danmalam U. H. Toxicity studies on the methanolic extract of Portulaca oleracea L. (Fam. Portulacaceae) Journal of Biological Sciences. 2007;7(7):1293–1295. doi: 10.3923/jbs.2007.1293.1295. [DOI] [Google Scholar]
- 243.Wasfi I. A., Bashir A. K., Abdalla A. A., Banna N. R., Tanira M. O. M. Antiinflammatory activity of some medicinal plants of the United Arab Emirates. Pharmaceutical Biology. 1995;33(2):124–128. doi: 10.3109/13880209509055211. [DOI] [Google Scholar]
- 244.Adam S. E. I. Experimental Rhazya stricta toxicosis in rats. Veterinary and Human Toxicology. 1999;41(1):5–8. [PubMed] [Google Scholar]
- 245.Stuart M. The Encyclopedia of Herbs and Herbalism. London, UK: Orbis Publishing; 1979. [Google Scholar]
- 246.Clause E. P., Varo E. T., Lynn R. B. Pharmacognosy. London, UK: Henry Kimpton; 1971. [Google Scholar]
- 247.Rafatullah S., Mossa J. S., Ageel A. M., Al-Yahya M. A., Tariq M. Hepatoprotective and safety evaluation studies on Sarsaparilla. International Journal of Pharmacognosy. 1991;29(4):296–301. doi: 10.3109/13880209109082901. [DOI] [Google Scholar]
- 248.Bomford R. Immunomodulators from plants and fungi. Phytotherapy Research. 1988;2(4):159–164. doi: 10.1002/ptr.2650020402. [DOI] [Google Scholar]
- 249.Nwaigwe C. U., Madubunyi I. I., Udem S. C., Nwaigwe C. O. Methanolic root extract of Olax viridis protects the liver against acetaminophen-induced liver damage. Research Journal of Medicinal Plant. 2012;6(5):395–405. doi: 10.3923/rjmp.2012.395.405. [DOI] [Google Scholar]
- 250.Drotman R. B., Lawhorn G. T. Serum enzymes as indicators of chemically induced liver damage. Drug and Chemical Toxicology. 1978;1(2):163–171. doi: 10.3109/01480547809034433. [DOI] [PubMed] [Google Scholar]
- 251.Raju K., Anbuganapathi G., Gokulakrishnan V., Rajkapoor B., Jayakar B., Manian S. Effect of dried fruits of Solanum nigrum Linn. against CCl4-induced hepatic damage in rats. Biological and Pharmaceutical Bulletin. 2003;26(11):1618–1619. doi: 10.1248/bpb.26.1618. [DOI] [PubMed] [Google Scholar]
- 252.Hsieh C.-C., Fang H.-L., Lina W.-C. Inhibitory effect of Solanum nigrum on thioacetamide-induced liver fibrosis in mice. Journal of Ethnopharmacology. 2008;119(1):117–121. doi: 10.1016/j.jep.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 253.Akula U. S., Odhav B. In vitro 5-lipoxygenase inhibition of polyphenolic antioxidants from undomesticated plants of South Africa. Journal of Medicinal Plants Research. 2008;2(9):207–212. [Google Scholar]
- 254.Cai X.-F., Chin Y.-W., Oh S.-R., Kwon O.-K., Ahn K.-S., Lee H.-K. Anti-inflammatory constituents from Solanum nigrum . Bulletin of the Korean Chemical Society. 2010;31(1):199–201. doi: 10.5012/bkcs.2010.31.01.199. [DOI] [Google Scholar]
- 255.Abd Elkawy M. M., Soliman G. Z. A., Abd-El Mobd'ea E., Abd-El Rehim Effect of Solanum nigrum Linn. against lambda cyhalothrin-induced toxicity in rats. IOSR-Journal of Pharmacy and Biological Sciences. 2013;5(5):55–62. doi: 10.9790/3008-0555562. [DOI] [Google Scholar]
- 256.Youssef A. M., Al-Fredan M. A., Fathi A. A. Floristic composition of lake Al-Asfar, Alahsa, Saudi Arabia. International Journal of Botany. 2009;5(2):116–125. doi: 10.3923/ijb.2009.116.125. [DOI] [Google Scholar]
- 257.Patra J. K., Dhal N. K., Thatoi H. N. In vitro bioactivity and phytochemical screening of Suaeda maritima (Dumort): a mangrove associate from Bhitarkanika, India. Asian Pacific Journal of Tropical Medicine. 2011;4(9):727–734. doi: 10.1016/S1995-7645(11)60182-X. [DOI] [PubMed] [Google Scholar]
- 258.Bandaranayake W. M. Traditional and medicinal uses of mangroves. Mangroves and Salt Marshes. 1998;2(3):133–148. doi: 10.1023/A:1009988607044. [DOI] [Google Scholar]
- 259.Orfali R. S. Phytochemical and Biological Study of Tamarix nilotica Growing in Saudi Arabia. King Saud University; 2005. [Google Scholar]
- 260.Shenoy S., Shwetha K., Prabhu K., Maradi R., Bairy K. L., Shanbhag T. Evaluation of antiinflammatory activity of Tephrosia purpurea in rats. Asian Pacific Journal of Tropical Medicine. 2010;3(3):193–195. doi: 10.1016/S1995-7645(10)60007-7. [DOI] [Google Scholar]
- 261.Hussain T., Fareed S., Siddiqui H. H., Vijaykumar M., Rao C. V. Acute and subacute oral toxicity evaluation of Tephrosia purpurea extract in rodents. Asian Pacific Journal of Tropical Disease. 2012;2(2):129–132. doi: 10.1016/S2222-1808(12)60030-9. [DOI] [Google Scholar]
- 262.Kalantari H., Forouzandeh H., Azemi M. E., Rashidi I., Goudarzi M. Study of the protective effect of Teucrium polium L. extract on acetaminophen-induced hepatotoxicity in mice. Iranian Journal of Pharmaceutical Research. 2013;12(1):123–129. [PMC free article] [PubMed] [Google Scholar]
- 263.Bomzon A., Shtukmaster S., Ljubuncic P. The effect of an aqueous extract of Teucrium polium on glutathione homeostasis in vitro: a possible mechanism of its hepatoprotectant action. Advances in Pharmacological Sciences. 2010;2010 doi: 10.1155/2010/938324.938324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Shakhanbeh J., Atrouse O. Teucrium polium inhibits nerve conduction and carrageenan-induced inflammation in the rat skin. Turkish Journal of Medical Sciences. 2001;31(1):15–21. [Google Scholar]
- 265.Capasso F., Cerri R., Morrica P., Senatore F. Chemical composition and anti-inflammatory activity of an alcoholic extract of Teucrium polium L. Bollettino della Societa Italiana di Biologia Sperimentale. 1983;59(11):1639–1643. [PubMed] [Google Scholar]
- 266.Abu Sitta K. H., Shomah M. S., Salhab A. S. Hepatotoxicity of ‘Teucrium polium’ L. tea: supporting evidence in mice models. Australian Journal of Medical Herbalism. 2009;21(4):106–109. [Google Scholar]
- 267.Soylu A. R., Sivri B., Bayraktar Y. Hepatoxicity of Teucrium polium . Turkish Journal of Gastroenterology. 1998;9(2):196–197. [Google Scholar]
- 268.Mattei A., Rucay P., Samuel D., Feray C., Reynes M., Bismuth H. Liver transplantation for severe acute liver failure after herbal medicine (Teucrium polium) administration. Journal of Hepatology. 1995;22(5):597–562. doi: 10.1016/0168-8278(95)80458-7. [DOI] [PubMed] [Google Scholar]
- 269.Baitar I. Jameul Mufradat al Advia wal Aghzia (Urdu Translation by CCRUM) 2nd. Vol. 2. New Delhi, India: CCRUM, Ministry of Health and Family Welfare; 2000. [Google Scholar]
- 270.Aguilar N. O. Trianthema portulacastrum L. Plant Resources of South East Asia 12(2): Medicinal and poisonous Plants 2. Backhuys; 2001. [Google Scholar]
- 271.Muthu C., Ayyanar M., Raja N., Ignacimuthu S. Medicinal plants used by traditional healers in Kancheepuram District of Tamil Nadu, India. Journal of Ethnobiology and Ethnomedicine. 2006;2, article 43 doi: 10.1186/1746-4269-2-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Kumar G., Banu G. S., Pappa P. V., Sundararajan M., Pandian M. R. Hepatoprotective activity of Trianthema portulacastrum L. against paracetamol and thioacetamide intoxication in albino rats. Journal of Ethnopharmacology. 2004;92(1):37–40. doi: 10.1016/j.jep.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 273.Mandal A., Karmakar R., Bandyopadhyay S., Chatterjee M. Antihepatotoxic potential of Trianthema portulacastrum in carbon tetrachloride-induced chronic hepatocellular injury in mice: reflection in haematological, histological and biochemical characteristics. Archives of Pharmacal Research. 1998;21(3):223–230. doi: 10.1007/BF02975279. [DOI] [PubMed] [Google Scholar]
- 274.Kumar G., Banu G. S., Pandian M. R. Evaluation of the antioxidant activity of Trianthema portulacastrum L. Indian Journal of Pharmacology. 2005;37(5):331–333. doi: 10.4103/0253-7613.16861. [DOI] [Google Scholar]
- 275.Vohora S. B., Shah S. A., Naqvi S. A. H., Ahmad S., Khan M. S. Studies on Trianthema portulacastrum . Planta Medica. 1983;47(2):106–108. doi: 10.1055/s-2007-969964. [DOI] [PubMed] [Google Scholar]
- 276.Asif M., Atif M., Malik A. S. A., Dan Z. C., Ahmad I., Ahmad A. Diuretic activity of Trianthema portulacastrum crude extract in albino rats. Tropical Journal of Pharmaceutical Research. 2013;12(6):967–972. doi: 10.4314/tjpr.v12i1.15. [DOI] [Google Scholar]
- 277.Kavitha P., Ramesh R., Bupesh G., Stalin A., Subramanian P. Hepatoprotective activity of Tribulus terrestris extract against acetaminophen-induced toxicity in a freshwater fish (Oreochromis mossambicus) In Vitro Cellular and Developmental Biology-Animal. 2011;47(10):698–706. doi: 10.1007/s11626-011-9457-9. [DOI] [PubMed] [Google Scholar]
- 278.Sambasivam M., Ravikumar R., Thinagarbabu R., Davidraj C., Arvind S. Hepatoprotective potential of Azima tetracantha and Tribulus terrestris on ferrous sulfate-induced toxicity in rat. Bangladesh Journal of Pharmacology. 2013;8(3):357–360. doi: 10.3329/bjp.v8i3.15579. [DOI] [Google Scholar]
- 279.Li J.-X., Shi Q., Xiong Q.-B., Prasain J. K., Tezuka Y., Hareyama T., Wang Z.-T., Tanaka K., Namba T., Kadota S. Tribulusamide A and B, new hepatoprotective lignanamides from the fruits of Tribulus terrestris: indications of cytoprotective activity in murine hepatocyte culture. Planta Medica. 1998;64(7):628–631. doi: 10.1055/s-2006-957535. [DOI] [PubMed] [Google Scholar]
- 280.Zheleva-Dimitrova D., Obreshkova D., Nedialkov P. Antioxidant activity of Tribulus terrestris—a natural product in infertility therapy. International Journal of Pharmacy and Pharmaceutical Sciences. 2012;4(4):508–511. [Google Scholar]
- 281.Baburao B., Rajyalakshmi G., Venkatesham A., Kiran G., Sunder A. S., Rao B. G. Anti-inflammatory and antimicrobial activities of methanolic extract of Tribulus terrestris Linn. plant. International Journal of Chemical Sciences. 2009;7(3):1867–1872. [Google Scholar]
- 282.Aslani M. R., Movassaghi A. R., Mohri M., Pedram M., Abavisani A. Experimental Tribulus terrestris poisoning in sheep: clinical, laboratory and pathological findings. Veterinary Research Communications. 2003;27(1):53–62. doi: 10.1023/A:1022010707704. [DOI] [PubMed] [Google Scholar]
- 283.Aslani M. R., Movassaghi A. R., Mohri M., Ebrahim-pour V., Mohebi A. N. Experimental Tribulus terrestris poisoning in goats. Small Ruminant Research. 2004;51(3):261–267. doi: 10.1016/S0921-4488(03)00195-0. [DOI] [Google Scholar]
- 284.Talasaz A. H., Abbasi M.-R., Abkhiz S., Dashti-Khavidaki S. Tribulus terrestris-induced severe nephrotoxicity in a young healthy male. Nephrology Dialysis Transplantation. 2010;25(11):3792–3793. doi: 10.1093/ndt/gfq457. [DOI] [PubMed] [Google Scholar]
- 285.Arcasoy H. B., Erenmemisoglu A., Tekol Y., Kurucu S., Kartal M. Effect of Tribulus terrestris L. saponin mixture on some smooth muscle preparations: a preliminary study. Bollettino Chimico Farmaceutico. 1998;137(11):473–475. [PubMed] [Google Scholar]