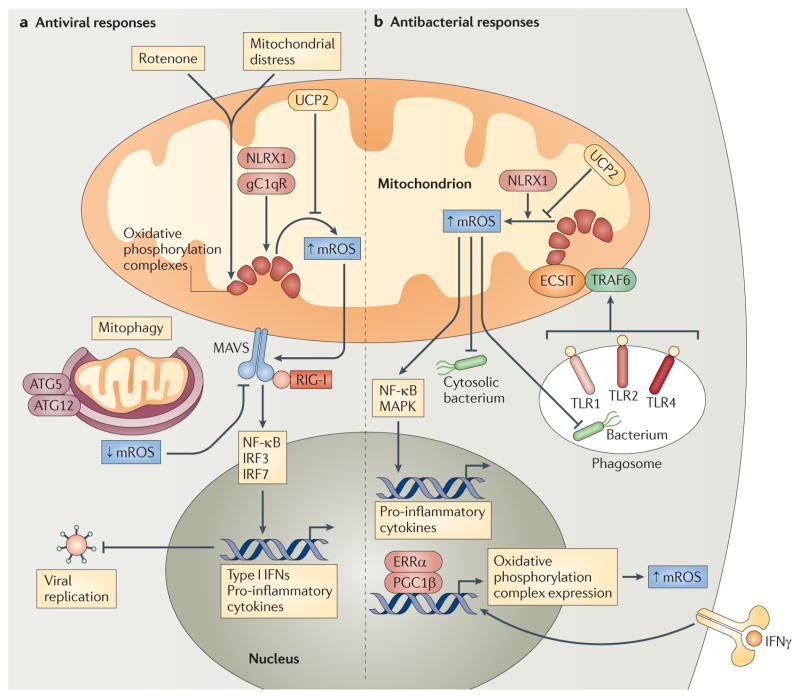
Figure 3. Mitochondrial ROS and innate immune responses.
a | Mitochondrial distress and/or rotenone treatment augment mitochondrial reactive oxygen species (mROS) generation from oxidative phosphorylation complexes, and this potentiates RIG-I-like receptor (RLR)–mitochondrial antiviral signalling protein (MAVS) signalling to nuclear factor-κB (NF-κB) and interferon regulatory factor 3 (IRF3) and IRF7. This increases the production of type I interferons (IFNs) and pro-inflammatory cytokines, which limit viral replication. NLR family member X1 (NLRX1) and receptor for globular head domain of complement component 1q (gC1qR) interact with oxidative phosphorylation complexes and may increase mROS generation, whereas uncoupling protein 2 (UCP2) decreases mROS production. Mitophagy mediated by autophagy-related gene 5 (ATG5) and ATG12 also decreases mROS production and subsequent RLR–MAVS signalling by removing dysfunctional, mROS-generating mitochondria. b | Phagocytized bacteria activate Toll-like receptor 1 (TLR1), TLR2 and TLR4, and this promotes the translocation of TNF receptor-associated factor 6 (TRAF6) to mitochondria, where it engages ECSIT (evolutionary conserved signalling intermediate in Toll pathways) to potentiate mROS generation from oxidative phosphorylation complexes. This leads to increased ROS-dependent bactericidal responses and/or activation of NF-κB and mitogen-activated protein kinase (MAPK) signalling to augment pro-inflammatory cytokine production. IFNγ signalling can also promote mROS generation and increased antibacterial innate immunity by engaging oestrogen-related receptor-α (ERRα) and the co-activator peroxisome proliferator-activated receptor-γ co-activator 1β (PGC1β), which upregulate mitochondrial biogenesis and the expression of nuclear-encoded oxidative phosphorylation genes. UCP2 is a negative regulator of mROS generation, whereas NLRX1 can enhance mROS production under certain circumstances. RIG-I, retinoic acid-inducible gene I.