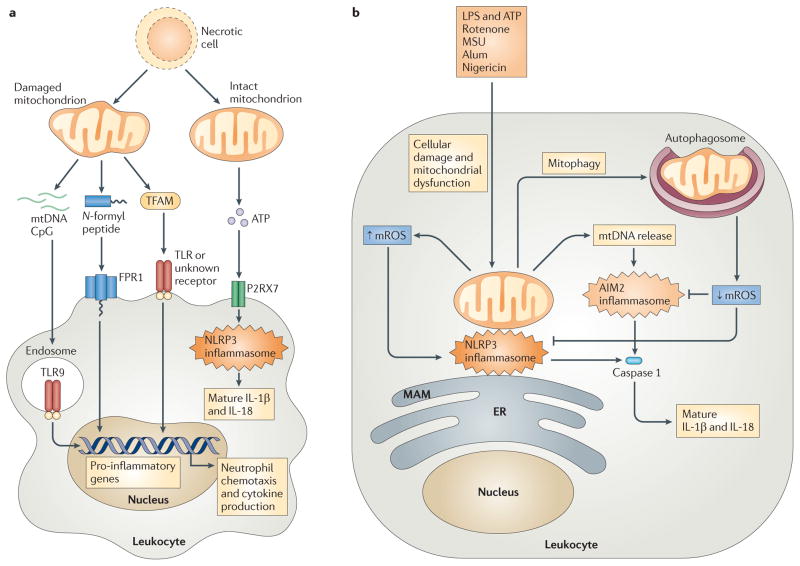
Figure 4. Mitochondrial involvement in cellular damage responses.
a | Cellular injury and necrosis release damaged mitochondria into the extracellular space, where they leak mitochondrial damage-associated molecular patterns (DAMPs) such as mitochondrial DNA (mtDNA), N-formyl peptides and mitochondrial transcription factor A (TFAM). Leukocytes, such as macrophages and neutrophils, detect mtDNA through Toll-like receptor 9 (TLR9), N-formyl peptides via formyl peptide receptor 1 (FPR1), and TFAM through TLRs or an uncharacterized receptor, leading to leukocyte activation and transcription of pro-inflammatory cytokine genes. Necrotic cells can also release intact mitochondria that secrete ATP. This ATP engages the leukocyte purinergic receptor P2RX7, which promotes NOD-, LRR- and pyrin domain-containing 3 (NLRP3) inflammasome activation and the subsequent processing and secretion of the cytokines interleukin-1β (IL-1β) and IL-18. b | Exposure of leukocytes to inflammasome activators — such as lipopolysaccharide (LPS) and ATP, rotenone, monosodium urate (MSU), alum and nigericin — leads to mitochondrial dysfunction and mitochondrial ROS (mROS) generation. mROS promote inflammasome activation at mitochondria-associated membranes (MAMs) of the endoplasmic reticulum (ER), by nucleating and activating a complex of NLRP3, ASC and caspase 1. This induces the proteolytic processing of pro-IL-1β and pro-IL-18 into mature and secreted forms. mROS also promote the release of mtDNA into the cytosol, and this DNA engages the absent in melanoma 2 (AIM2) inflammasome or other receptors to further augment IL-1β and IL-18 processing and secretion. Mitophagy serves as a brake on inflammasome activation by mediating the clearance of damaged, mROS-generating mitochondria.