Abstract
Understanding of cAMP signaling has greatly improved over the past decade. The advent of live cell imaging techniques and more specific pharmacologic modulators has led to an improved understanding of the intricacies by which cAMP is able to modulate such a wide variety of cellular pathways. It is now appreciated that cAMP is able to activate multiple effector proteins at distinct areas in the cell leading to the activation of very different downstream targets. The investigation of signaling proteins in cancer is a common route to the development of diagnostic tools, prognostic tools, and/or therapeutic targets, and in this review we highlight how investigation of cAMP signaling microdomains driven by the soluble adenylyl cyclase in different cancers has led to the development of a novel cancer biomarker. Antibodies directed against the soluble adenylyl cyclase (sAC) are highly specific markers for melanoma especially for lentigo maligna melanoma and are being described as “second generation” cancer diagnostics, which are diagnostics that determine the ‘state’ of a cell and not just identify the cell type. Due to the wide presence of cAMP signaling pathways in cancer, we predict that further investigation of both sAC and other cAMP microdomains will lead to additional cancer biomarkers. This article is part of a Special Issue entitled: The role of soluble adenylyl cyclase in health and disease.
Keywords: Soluble adenylyl cyclase, cAMP, Microdomain, Cancer, Diagnostics
1. Introduction
Cyclic adenosine monophosphate (cAMP) is one of the most ancient signaling molecules present from bacteria to man. In mammals, cAMP controls a wide range of cellular processes and is present in every cell type and organ. cAMP is synthesized from ATP by a class of enzymes called adenylyl cyclases (ACs), which are encoded by 10 different genes (ADCY1–10) [1]. ACs 1–9 encode for proteins with a fairly similar structure in that all of them are transmembrane proteins (tmACs) and reside principally at the plasma membrane and endosomes making these ACs well suited to respond to extracellular signals. tmACs provide an important link between hormonal (e.g., melanocortin stimulating hormone) signals and intracellular processes. In many ways, tmACs function to coordinate cells within a tissue. Most tmACs are principally regulated by G protein coupled receptors via direct stimulation by heterotrimeric G proteins either by direct interaction between tmACs and the G s subunit or subunits [1]. Regulation of tmACs can be divided into four groups: Group 1, Ca2+/calmodulin-stimulated AC1, AC3, and AC8; Group 2, G -stimulated and Ca2+-insensitive AC2, AC4, and AC7; Group 3, G i/Ca2+/PKA-inhibited, AC5 and AC6; and Group 4, forskolin/Ca2+/ G -insensitive, AC9 [1]. The more recently identified AC (ADCY10) is also called the soluble adenylyl cyclase (sAC), which unlike the tmACs has no membrane spanning motifs and therefore is free to localize to multiple locations within a cell of which the best characterized are the nucleus and mitochondria [2,3]. sAC is primarily regulated by changes in bicarbonate [4] and calcium ions [5]. Bicarbonate ion functions to both increase the Vmax of the enzyme and alleviate substrate, ATP, and inhibition [5]. The ability to sense bicarbonate allows sAC to function as a pH sensor [2,6]. Calcium functions to decrease the Km for MgATP [5]. Whereas most proteins have a Km for MgATP that far exceeds the normal resting levels of ATP in the cell, ~1–3 mM (e.g., tmACs have a Km for MgATP in tens to hundreds of micromolar [7]), sAC's Km in the presence of calcium is approximately 1–3 mM. The elevated Km for MgATP enables sAC to sense changes in metabolism [8,9]. In addition to regulation by bicarbonate, calcium and ATP, the sAC protein contains a P loop [10], a heme binding domain [11] and other predicted protein domains and phosphorylation sites that may provide additional regulatory mechanisms. As a pH and metabolic sensor, sAC is poised to function as an intrinsic sensor of cellular health.
Since sAC and the nine different tmACs each respond to distinct signals yet produce the same second messenger, cAMP, it is important for the cell to respond specifically to each source of cAMP. The cell has at its disposal three families of cAMP effector proteins, an entire family of cAMP catabolizing enzymes, and a family of scaffolding proteins allowing the cell to establish spatially and temporally separate cAMP signaling domains (microdomains) capable of inducing a wide variety of downstream cascades. cAMP microdomains were first appreciated in the 1970s by the groups of Keely, Hayes, Brunton, and others when they recognized that different tmAC activating hormones (e.g., β-adrenergic receptor and prostaglandin E1 agonists) all led to cAMP elevation but each induced unique cellular events in cardiomyocytes, e.g., only β-adrenergic stimulation induced increased contractility and glycogen metabolism [12].
In the following sections, we will review the role of exchange protein activated by cAMP, protein kinase A, and A kinase anchoring proteins in cAMP signaling and how investigations of each have contributed to our understanding of cAMP microdomains. For the purpose of brevity, we have chosen not to review the vast literature of cyclic AMP gated ion channels, which lie at the plasma membrane and are an important link between cAMP signaling and ion transport, and phosphodiesterases, which catabolize cAMP into AMP and can be localized to many different areas of the cell. Finally, we will discuss cAMP signaling in cancer, specifically melanoma, and how a better understanding of cAMP microdomains in cancer may improve cancer diagnostics.
2. Exchange protein activated by cAMP
The exchange protein activated by cAMP (EPAC) family of effector proteins was discovered coincidently by two laboratories in 1998. One group initiated a database search to determine how cAMP could activate the small G protein Rap1 in a PKA-independent manner [13] while the other group found both EPAC1 (cAMP-GEF-I) and EPAC2 (cAMP-GEF-II) in a differential display screen for novel cAMP binding proteins [14] . Small G proteins cycle between a GDP bound inactive state and a GTP bound active state. Proteins such as guanine nucleotide exchange factors (GEFs) facilitate the exchange of GDP for GTP whereas GTPase-activating proteins (GAPs) help G proteins convert GTP back to GDP. Because small G proteins have a very slow intrinsic GTPase activity, restricted access to GAPs leads to prolonged activation of the proteins, explaining why Ras activating mutations block association of Ras with GAPs. Therefore, proper control of the Rap1 and Rap2 cascades require both spatial and temporal control over the GEFs and GAPs. EPAC can have multiple splice variants and is broadly expressed depending on developmental stage and disease [15]. EPAC1 is highly expressed in the heart, kidneys, blood vessels, adipose tissue, central nervous system, ovaries, and uterus [14,16] with multiple hematopoietic cell types. EPAC2, however, is mostly expressed in the central nervous system, adrenal gland, and pancreas with no detectable expression in hematopoietic cells [17]. EPACs are multidomain proteins with regulatory domains at the N terminus and the catalytic GEF domain at the C terminus. Both EPAC genes encode for cAMP binding domains (cAMP-B domain in EPAC1 and both cAMP-A and B domains in EPAC2), a disheveled-Egl-10-pleckstrin (DEP) domain that is responsible for plasma membrane localization, a Ras exchange motif (REM) domain that assists in catalysis, a Ras association (RA) domain that allows for interaction with GTP bound G proteins (e.g., Ras), and the CDC25HD domain that provides GEF activity [17]. Binding of cAMP to the cAMP-A and/or B domains along with interaction with small G proteins via the RA domain can alter the location of EPAC proteins and control when and where Rap1 is activated [15,18]. Interestingly, EPAC1 is known to associate with Ran-GTP, which is thought to control its nuclear localization. Nuclear EPAC1 is thought to control the DNA damage response and the DNA damage-responsive DNA-protein kinase [17]. EPAC controls nuclear export of histone deacetylase (HDAC)−4 and −5 [19,20]. The presence of EPAC in the nucleus predicts that a source of cAMP should exist to regulate its activity and/or localization. sAC was shown to localize to the nucleus in numerous cell types by cellular fractionation and immunohistochemistry [3] and later confirmed by FRET based imaging [21]. sAC moves in and out of the nucleus of certain cells during specific inflammatory diseases (e.g., psoriasis), infectious diseases (e.g., human papillomavirus infection), and malignancy (e.g., squamous cell carcinoma and melanoma) discussed in more detail below [3,22,23]. Since sAC is known to regulate Rap1 in an EPAC-dependent manner it is plausible that sAC is a nuclear source of cAMP regulating EPAC in the nucleus. However, it is important to note that some groups have found that activation of tmACs can also lead to a rise in nuclear cAMP as measured by a EPAC-FRET probe [21].
3. Protein kinase A
Protein kinase A (PKA) is one of the first characterized and best known cAMP effector proteins. It is a broad specificity serine/threonine kinase that consists of two catalytic (C) and two regulatory (R) subunits. This tetrameric holoenzyme is activated when two molecules of cAMP bind to each R subunit resulting in a confirmational change and release of active C subunit [24]. There are three C subunits (α, β, and γ). Cα and Cβ are ubiquitously expressed but Cγ is expressed mainly in the testis [25]. There are four R subunits which fall into two categories: RI (α and β) and RII (α and β), and all isoforms are able to bind cAMP. Each isoform has unique protein domains, which target isoforms to distinct areas of the cell, and therefore, are subject to different regulation and downstream substrates [24–26]. Regulatory subunits are not functionally redundant. The two major stable and well-folded domains are the dimerization/docking domain at the N terminus and the two tandem cAMP binding domains at the C-terminus. The D/D domain is a four-helix bundle that binds to the amphipathic helix motif characteristic of A kinase anchoring proteins (AKAPs), which will be discussed in more depth in the next section [27]. A number of studies have identified PKA at distinct locations throughout the cell including the mitochondria [13], the nucleus [28], and the centriole [29], in addition to the plasma membrane. Localization of PKA is driven by the association of PKA with AKAPs.
4. A kinase anchoring proteins
AKAPs are a large and diverse group of proteins defined by their ability to bind PKA [25]. There are 43 known genes, which encode the AKAP family of proteins [30] and many of these genes produce mRNAs that are alternatively spliced thereby producing >70 distinct AKAP proteins. As mentioned above, AKAPs are able to bind to the R subunit of PKA via an amphipathic helix consisting of 14–18 amino acids that bind to the D/D domain on R subunit [31]. Originally AKAPs were thought to only bind to RII subunits but it is now accepted that RI subunits can also bind albeit with much lower affinity [31,32]. AKAPs are found throughout the cell in many different organelles and associated with different proteins. This allows AKAPs to tether PKA to different areas of the cell thereby directing cAMP signaling to specific targets. While AKAPs are defined by the ability to bind PKA they can also form multimolecular signaling complexes, which can include other cAMP signaling proteins such as PDEs and EPAC, and/or proteins from other signaling cascades such as MAPK signaling proteins [33]. These AKAP defined microdomains have been well established in cardiac myocytes and the role of AKAPs in these cells and others has been reviewed previously [33,34]; however, we will illustrate specific examples of how AKAPs define cAMP microdomains to highlight how cAMP can signal in unique areas of the cell.
4.1. AKAPs in the nucleus
AKAP95 is a nuclear AKAP, which is known to bind the RII subunit of PKA [35]. It can also bind to cyclin E1 and this interaction is displaced by cyclin dependent kinases. Interaction of G1/S cyclins can also interact with RII via AKAP95 suggesting that AKAP95 has a broader effect on the cell cycle providing a focal point for the interaction between the cell cycle and cAMP signaling [36]. AKAP95 is associated with the nuclear matrix during interphase and is found mostly in the chromatin fraction during mitosis. During mitosis, AKAP95 brings PKA to the chromatin and PKA activity is important for the maintenance of condensed chromatin [37]. AKAP7γ is another nuclear AKAP found in both HEK 293 cells and oocytes and is a dual-specific AKAP binding both RI and RII subunits of PKA [38]. It is currently unknown what function is performed by AKAP7γ defined microdomains. Another AKAP, AKAP150, which binds to RII, is found in the nucleus of precartilidge cells but is virtually absent from differentiated chondrocytes. This implies that a nuclear AKAP/PKA microdomain is important for mesenchymal cells prior to chondrogenesis [39] and highlights how the same cell in different stages of growth or differentiation can have different requirements for nuclear cAMP. The source of cAMP regulating PKA in the nucleus has been the subject of debate. We and others have demonstrated the presence of a sAC-dependent cAMP microdomain in the nucleus capable of regulating nuclear PKA [3,40] whereas others have shown that activation of tmACs leads to a delayed rise of cAMP in the nucleus and PKA activation [41]. It remains uncertain whether the nuclear rise of cAMP following tmAC activation at the plasma membrane is due to diffusion of cAMP from the plasma membrane or the creation of secondary signaling pathways (e.g., calcium) capable of leading to sAC activation of PKA in the nucleus.
4.2. AKAPs at the centriole
One of the earliest identified AKAPs was found localized to the centriole, the cellular structure responsible for organization of microtubules during mitosis. Pericentrin is an integral component of the pericentriolar matrix of the centrosome and is important for centrosome assembly and organization [42]. PKA RII associates with pericentrin. Another centrosomal AKAP is AKAP9/450/350/DG-NAP was recently shown to be part of a multimeric complex consisting of PKA, PDE4D3, and AKAP9. PDE4D3 regulates a dynamic cAMP microdomain at this AKAP that changes over the cell cycle [29].
4.3. AKAPs at and in the mitochondria
Mitochondria have been investigated for cAMP microdomains on the surface and within the organelle. The role of cAMP-dependent signaling cascades, namely those defined by the soluble adenylyl cyclase, which are present within the mitochondria will not be reviewed here. In this review, we will identify a few examples of AKAPs that are present at and within the mitochondria and how each defines a distinct cAMP microdomain within a single organelle. SKIP is an RI-specific AKAP, which was shown to localize to the intermembrane space of the mitochondria and targets PKA to phosphorylate the protein ChChd3, a protein essential for maintaining cristae integrity [43]. Another mitochondrial AKAP is Rab32, a member of the Ras superfamily of small G proteins. Rab32 is a dual function protein both involved in PKA anchoring and mitochondrial fission. Proper function of Rab32 is important for movement of mitochondria around the microtubule organizing center [44]. AKAP121 is a unique mitochondrial AKAP in that it binds both PKA and steroidogenic acute regulatory protein (STAR) mRNA, which permits efficient translation and phosphorylation of STAR protein. AKAP121 is thought to enhance steroidogenesis at the mitochondria in response to cAMP by directing the synthesis and activation of STAR [45]. AKAP121 has been identified both outside and on the inner compartment of mitochondria [46]. AKAP121 is important for cardiac health and its loss leads to the neonatal cardiomyopathy [47].
5. cAMP microdomains in cancer
As we have discussed above, there exist multiple cAMP effector proteins and cAMP regulatory proteins and these proteins exist in microdomains throughout the cell. cAMP microdomains can work in concert [9] or have opposing downstream effects [48]. This can sometimes make the study of cAMP signaling confusing, and this fact is no better highlighted than in cancer biology. In the next section we will review some examples of how cAMP signaling can have widely opposing effects in the deadly skin cancer melanoma. The prototypical cAMP pathway in melanoma begins with the activation of a G protein coupled receptor such as the melanocortin 1 receptor (MC1R) by the binding of a ligand such as melanocortin stimulating hormone (MSH). MC1R activation leads to stimulation of tmACs and the generation of cAMP. cAMP plays an important role in melanocyte biology as a regulator of growth and pigmentation. Under normal growth conditions, activation of tyrosine kinase receptors leads to stimulation of Ras and subsequent activation of BRAF, MEK, and ERK. While melanocytes contain both BRAF and CRAF kinases, Ras preferably activates BRAF in normal melanocytes mainly because CRAF is kept in an inactive state due to phosphorylation by PKA. Interestingly, and for reasons not completely understood, when NRAS is mutated in melanoma and becomes activated, the preferred RAF kinase becomes CRAF [49,50]. This isoform switching is the result of PDE4 activation and the inhibition of cAMP [51]. These data suggest that cAMP is an anti-proliferative signal for melanoma and is consistent with the hypothesis that reactivation of cAMP signaling in NRAS mutant melanoma will induce apoptosis [51]. However, there are also examples of cAMP elevation having growth promoting effects in melanoma. BRAFV600E is a common driver mutation for melanoma and while targeted therapy for this protein exists, resistance soon develops. The Garraway group recently published an unbiased analysis of cDNAs capable of rescuing the inhibition of BRAFV600E. Of the 110 cDNAs examined, many of the proteins capable of rescuing cell death were cAMP signaling proteins. These data suggest that under these circumstances cAMP activation promotes melanoma growth. These two examples highlight how a more complete understanding of cAMP signaling is needed to better understand melanoma. Furthermore, since melanoma (regardless of driver mutation) and melanocytes contain the same cAMP signaling proteins it is reasonable to hypothesize that these disparate cAMP-dependent effects are driven by distinct microdomains.
The recurrent observation in melanoma and other cancers that cAMP could play so many seemingly distinct roles prompted us to examine cAMP microdomains in cancer. We were reminded of earlier data that sAC driven cAMP microdomains can localize to both the cytoplasm, mitochondria and nucleus and that this localization of sAC is transient [3,22]. We and other investigators have examined whether sAC is found in distinct localizations in different cancers and whether these patterns are the same in benign tissue. Since sAC is known to impact multiple aspects of cellular biology, might a specific localization of sAC or change in localization be a helpful marker for clinicians? We will discuss ongoing studies to address these questions.
6. Use of R21 in the evaluation of the normal epidermis and keratinocyte proliferations
The laboratory of Drs. Lonny Levin and Jochen Buck developed a cadre of monoclonal antibodies, which have proven useful for the study of cAMP microdomains in human tissue [22,28]. We will focus on one of those antibodies called R21. In the original study of R21 in human tissue by Zippin et al. [22], cases of normal skin, virally infected skin, benign and malignant epidermal neoplasia, and inflammatory-mediated epidermal proliferations were stained with the mouse monoclonal antibody against human sAC protein. In normal skin, sAC was expressed diffusely in the keratinocytes without specific organellar localization. Rare keratinocyte nuclear staining was noted. No staining was identified in the stratum corneum. Additionally, T-cells, macrophages, dendritic cells, eccrine ducts, and cutaneous nerve axons also expressed sAC. Cases of verruca vulgaris revealed significantly reduced cytoplasmic staining with localization to the nucleus in six of nine cases. Occasional nuclei lacked staining. In contrast to verruca vulgaris, all seven cases of molluscum contagiosum lacked nuclear staining and instead exhibited granular cytoplasmic and perinuclear staining thought to be mitochondrial. Interestingly, high grade squamous dysplasia (i.e. bowenoid papulosis) associated with high-risk strains of HPV (types 16, 18, 31, and 33) lacked nuclear expression and instead demonstrated perinuclear granular staining. Seborrheic keratoses, actinic keratoses, and UV-associated squamous cell carcinoma in situ exhibited predominantly nuclear sAC staining with decreased cytoplasmic staining compared to normal epidermis. Interestingly, approximately 50% of invasive squamous cell carcinomas exhibited nuclear localization of sAC within the in situ component, but lost nuclear expression in the invasive component. In fact, in some of these invasive foci, there was complete loss of sAC staining. Compared with squamous cell carcinoma, basal cell carcinomas demonstrated a very different sAC immunostaining pattern in which intense cytoplasmic staining and no nuclear staining was identified. In inflammatory lesions, sAC localized to the nucleus in all forms of psoriasis, whereas in pityriasis rubra pilaris the staining was predominantly cytoplasmic with little to no increase in nuclear staining. Nuclear staining was further investigated in vitro in the cell line MDCK. It was established that sAC was present in the nucleus of actively dividing cells but was excluded from the nucleus in differentiated cells [22].
The observation that sAC staining is different between benign and malignant keratinocytes prompted the investigation of other cell types and cancers namely melanoma. Because of the difficulty in diagnosing melanoma and the severity of the disease, the application of sAC antibodies to the evaluation of pigmented lesions has become an area of active research. To properly appreciate how investigation of cAMP microdomains has added to the diagnosis of cancer, we will first review the current state of melanoma diagnostics and then focus on the data supporting the use of anti-sAC antibodies in the diagnosis of melanoma.
7. Current state of melanoma diagnostics
Malignant melanoma is among the most aggressive of human malignancies, with incidence rising 3–5% per year in the young and elderly populations. As its incidence continues to rise, it is estimated that by 2015, one in 50 Americans will develop melanoma during their lifetime [52]. Accurate and early diagnosis is crucial for prevention of disease spread, and is a significant factor in determining survival.
7.1. Diagnostic interpretation of melanocytic proliferations
Routine light microscopy in which architectural and cytologic features are independently assessed to formulate a prediction of biological behavior remains the gold standard for distinguishing benign and malignant melanocytic proliferations [53]. In benign melanocytic nevi, rounded nevomelanocytes proliferate along the dermal epidermal junction forming nests at the tips of the rete ridges termed a junctional nevus. Melanocytes may also be present in the dermis but exhibit so-called maturation with descent where the cells become smaller and more neuroidal in morphology thereby forming a compound nevus. Dermal mitoses should not be present. Cases in which all of the nevus cells are confined to the dermis and maintain maturation with descent are intradermal nevi. Cytologically, these intraepidermal and dermal nevomelanocytes lack significant pleomorphism with small round nuclei and inconspicuous nucleoli. Loss of melanocyte cohesion and upward scatter into the epidermis combined with areas of confluent growth are features consistent with superficial spreading melanoma in situ. Nests of melanocytes may be seen infiltrating the dermis in invasive melanoma where the melanocytes reveal little evidence of maturation with descent and occasional mitoses. These malignant melanocytes are typically large and pleomorphic with prominent nucleoli. The dys-plastic nevus is a melanocytic proliferation characterized by a junctional melanocytic component that extends laterally beyond the dermal component (so-called “shoulder”), fused rete ridges via nests of nevomelanocytes, and drape-like fibroplasia within the papillary dermis. The fusion of the rete ridges by nevomelanocytes may focally demonstrate confluent melanocytic growth and occasional rare melanocytes may be seen above the basal layer. Additionally, the lateral junctional “shoulder” of melanocytic nests may result in lesional asymmetry, which may signify additional mutations and evolution to melanoma. Many dermatopathologists believe these lesions pose a risk for biological instability and therefore a grade of cytologic atypia is reported along with the diagnosis [54]. Unfortunately, the unequivocal distinction between benign nevus and malignant melanoma cannot be made in some cases, in which case re-excision is performed.
7.2. Immunohistochemical (IHC) stains in the diagnosis of melanoma
With the rapid evolution of this malignancy, there is a great need for improving diagnostic techniques and IHC evaluation is the mainstay of adjunctive diagnostic techniques. Melanoma has a wide variety of histopathological presentations, making it difficult to rely on pathological features alone to make an accurate diagnosis. In addition, metastatic melanoma can be found anywhere in the body and can present with a myriad of signs and symptoms. Thus, while conventional histology is the gold standard for diagnosis of melanoma, it often fails to accurately distinguish benign from malignant melanocytic lesions and to correctly identify metastatic melanocytic lesions. IHC studies are frequently utilized to aid in the diagnosis of melanoma, particularly melanocytic differentiation and proliferation markers. However, while our current armamentarium of IHC markers is able to differentiate melanocytic from non-melanocytic lesions, they are less apt at distinguishing dysplastic nevi from malignant neoplasms. Identification of new immunohistochemical markers would greatly expand the diagnostic efforts of clinicians and dermatopathologists and improve survival among patients with melanoma.
7.3. Melanocyte identification markers
Among the myriad of IHC markers identified, melanocytic markers have been successfully utilized in the distinction of melanocytic tumors from non-melanocytic histologic mimics. Of these markers, S-100 remains one of the oldest and most frequently used melanocytic markers in clinical practice. Originally extracted from a protein fraction in bovine brain tissue in 1965, S-100 is a calcium-binding protein soluble in 100% saturated ammonium sulfate [55]. The S-100 protein family has greater than 25 members and is encoded by several genes many of which are located on the “epidermal differentiation cluster” in 1q21. This area is frequently rearranged in many tumors, including melanoma [56]. S-100 proteins are multifunctional, participating in a variety of cellular processes including cell growth and differentiation, cell cycle progression, structural membrane organization, transcription, motility, protein phosphorylation and protection from oxidative cell damage [57]. These proteins have been identified in Schwann cells, astrocytes, oligodendrocytes, adrenal medulla, chondrocytes, adipocytes, myoepithelial cells and melanocytes [58].
Expressed in more than 95% of primary cutaneous melanomas, S-100 is the most sensitive marker for melanocytic lesions, with sensitivity reported from 88% to 100% [56,59]. In addition, S-100 is one of the few immunohistochemical markers to identify spindle cell melanomas, though 10% of desmoplastic melanomas have weak or no staining with S-100 [23,60]. S-100 can be detected with rabbit or mouse monoclonal or polyclonal antibodies that react strongly with S-100B primarily, and weakly with S-100A1 or S-100A6 [61]. The characteristic pattern of S-100 in melanoma is strong and diffuse staining in both the nucleus and cytoplasm, with some areas of focal staining as well [62]. While S-100 is a frontrunner in sensitivity, the abundant and varied expression of this protein limits its specificity for identifying malignant melanocytic lesions, reported at 70–87% [63,64]. S-100 even reacts with certain epithelial neoplasms, including mammary carcinoma and other carcinomas of the salivary glands, sarcomas and Langerhans cell histiocytosis. Therefore, while S-100 remains a useful immunohisto-chemical marker for melanocyte identification, its use is limited when distinguishing between benign and malignant lesions.
HMB (human melanoma black) 45 is another useful marker of melanocyte identification used in the histopathological diagnosis of melanoma. This marker identifies the cytoplasmic premelanosomal glycoprotein gp100 (PMEL17, SILV, ME20, D12S53E), a type 1 membrane-bound melanosomal protein product of the SILV gene, which plays an important role in the structural organization of melanosomes [65]. HMB45 is a monoclonal antibody raised against the whole cell extract of the pigmented melanoma cell line Mel-1, derived from a lymph node involved in metastasis [66]. The HMB immunogen reacts with cutaneous fetal melanocytes and prenatal, infantile retinal pigmented epithelium, but does not react with adult normal melanocytes [65]. HMB45 stains junctional nevi and the junctional component of compound nevi, but does not stain intradermal nevi and the deep component of compound nevi [67]. Numerous studies have reported HMB45 sensitivity as 69–95% and specificity of up to 100% for diagnosis of melanoma; however, HMB45 is known to be less sensitive in metastatic melanoma [61,68, 69]. Thus, this marker can be helpful in distinguishing primary cutaneous melanoma from some nevi, especially in conjunction with the proliferation marker Ki67 [70].
HMB45 has a distinct cytoplasmic staining pattern in melanocytic lesions [71]. In benign nevi, HMB45 labeling is strongest at the upper portion of the lesion, but is decreased, with limited or no signal in the deeper dermis; in contrast, in primary cutaneous melanoma, HMB-45 staining is patchy in both the superficial and deep portions of the lesion [70]. Due to the patchy staining in intraepidermal melanocytes, studies have noted that HMB45 often underestimates melanocyte density in these lesions, and fails to detect all desmoplastic and many amelanotic melanomas, posing potential diagnostic problems with this marker [67]. Aside from melanocytic lesions, HMB45 also reacts with PEComas, clear cell sarcomas, pigmented neuroectodermal tumors, ovarian steroid cell tumors and renal cell sarcomas with t(6;11)(p21;q12) translocations, as well as some breast and sweat gland tumors [61]. HMB45 is therefore useful in the diagnosis of some cutaneous melanomas, but has some drawbacks.
Another useful marker of melanocyte identification is Melan-A, a product of the MART-1 gene located on chromosome 9p24.1 [63]. Melan-A is a transmembrane protein cloned from the melanoma cell line SK-Mel29 that is recognized by autologous cytotoxic T-cells [72]. The protein plays a role in stability, expression and trafficking of the protein PMEL17, and is important in the formation of stage II melanosomes [64]. Melan-A is expressed in the cytoplasm of mature melanocytes of the skin and retina and is localized to the melanosomes and endoplasmic reticulum. The overall sensitivity of Melan-A is 85–95% in primary melanoma and 57–92% in metastatic melanomas [73,74].
Two mouse monoclonal Abs were created to identify Melan-A is tissue. M2-7C10 was produced by Kawakami et al. at the National Cancer institute, and A103 was produced by Chen et al. at the Ludwig institute [63,72]. Aside from melanomas, these antibodies stain junctional, intradermal, compound, congenital, Spitz and dysplastic nevi limiting its specificity [64]. Interestingly, Melan-A stains the dermal component of nevi homogenously, while other markers such as HMB45 and PNL2 stain the dermal–epidermal and superficial junction of melanocytes [70]. To its disadvantage, Melan-A stains pigmented keratinocytes, and falsely labels pseudomelanocytic nests in the setting of a lichenoid infiltrate, further limiting its specificity [65]. Furthermore, this protein is not expressed in desmoplastic melanomas. Aside from melanocytic lesions, Melan-A reacts with PEComas, some clear cell sarcomas, some adrenal cortical and gonad steroid tumors (A103) [75]. One significant advantage of Melan-A over other markers is its lack of expression in dendritic cells in lymph nodes. This feature makes Melan-A a useful marker in detection of micrometastasis in sentinel lymph nodes, superior to S100, which does stain dendritic cells [73]. Melan-A is another useful marker of melanocyte identification in the diagnosis of primary cutaneous and metastatic melanoma.
Microphthalmia transcription factor (MITF) is a bHLH-Zip dimeric transcription factor encoded by the MITF gene on chromosome 3p14.1. MITF exists in 10 isoforms, of which MITF-M is selectively expressed in melanocytes and regulates transcription of genes for melanogenesis, cell survival and differentiation [69]. A monoclonal antibody to MITF, D5, is used to detect the nuclear pattern of protein expression in tissues. This nuclear staining greatly facilitates diagnosis in cases of suspected melanoma with cytoplasmic pigment. MITF is expressed in melanoma as well in as all common, acquired, dysplastic, blue and Spitz nevi [69]. In numerous studies, MITF has a reported sensitivity of 81–100%, with a specificity of 88–100% for malignant melanoma [62,70,74]. Significantly lower sensitivity is reported in desmoplastic lesions, ranging from 0 to 55% [69,76]. MITF can be particularly challenging in suspected cases of desmoplastic melanoma, as it stains mesenchymal and neural spindle cell neoplasms, as well as Schwann cells and stromal fibroblasts [69]. Other non-melanocytic lesions, including angiomyolipomas, clear cell sarcomas, lymphangiomas and reports of carcinomas of the pancreas, kidney, breast and ovary express MITF, decreasing the specificity of this marker for melanoma [74].
A newer immunohistochemical marker, PNL2, has been less frequently used in the diagnosis of melanocytic lesions. PNL2 is a monoclonal antibody generated against a subtype of the human somatostatin receptor SST2. While the specific antigen is unknown, the protein likely plays a role in melanin pigment synthesis or storage [70]. PNL2 is expressed in melanocytes and neutrophils, staining benign nevi and primary malignant melanoma, Spitz, dysplastic and blue nevi. In compound nevi, staining is most prominent at the dermal–epidermal junction and superficial dermis, whereas the deep dermal component is often negative or very weakly positive [70, 71]. PNL2 reportedly stains 80% of spindle cell melanomas, but does not stain desmoplastic melanomas or other non-melanocytic soft tissue neoplasms, with the exception of the pigmented component of melanotic schwanomas and PEComas [70]. This melanocytic marker has a reported sensitivity similar to that of Melan-A, MITF and Tyrosinase at 89%, but was found to recognize some S100-negative metastatic melanomas, making it potentially useful as an adjunct to S100 in immunohistochemical studies [71].
KBA62 is another newer melanocytic marker raised to the KAL melanoma cell line. This marker recognizes an unknown antigen expressed in melanoma cell lines and in malignant melanoma and some carcinomas [71]. Unlike other melanocytic markers, KBA62 also detects pericytes and stains vascular and visceral smooth muscle, breast myoepithelial, epidermal basal and stromal cells, as well as the glomerular basement membrane, hair shaft of the epithelia of skin, astrocytes, and cells of the uterine exocervix and tonsils [77]. In melanocytic lesions, KBA62 reacts with all histopathologic subtypes of nevi, staining the deep dermal component of compound nevi, unlike PNL2, which does not stain the deeper dermal area [70]. KBA62 identified primary, metastatic and desmoplastic melanoma with 93%, 86–91% and 82–100% sensitivity, respectively [70,71].
Similar to Melan-A, KBA62 does not react with dendritic cells in lymph nodes, making it a helpful marker in investigated sentinel lymph node metastasis [73]. Non-melanocytic lesions including clear cell sarcomas and a variety of carcinomas, neuroectodermal and mesynchymal neoplasms have shown to be reactive, while PEComas are usually negative [71].
7.4. Melanocyte proliferation markers
While melanocytic identification markers distinguish melanocytic lesions from non-melanocytic neoplasms, proliferation markers attempt to separate malignant lesions from their benign counterparts. Of the proliferation markers studied, Ki67 is the most widely used by dermatopathologists. Ki67, detected by the monoclonal antibody MIB-1, is expressed in the nuclei during G1, M, G2 and S phase of the cell cycle, but is absent in G0 and resting cells [78]. Studies have shown little to no staining in common benign nevi, but significantly increased staining in primary and metastatic melanomas [79]. Patterns of expression in melanocytic lesions reflect cellular maturation; while common and dysplastic nevi show immunoreactivity in less than 1% of cells, specifically at the dermal–epidermal junction or superficial dermal, melanomas show diffuse, random reactivity in more than 10% of cells [59]. A similar pattern is identified when using HMB45 to investigate a melanocytic lesion [59]. Furthermore, staining patterns in malignant neoplasms suggest that proliferative activity may be related to degree of malignancy, as higher levels of MIB-1 expression have been identified in metastatic melanomas [78,80]. Proliferation index seems to correlate with prognostic parameters such as Breslow thickness, nuclear size and tumor stage as well [81]. Ki67, in conjunction with melanocytic identification markers, is an important immunohisto-chemical marker for the diagnosis of primary cutaneous and metastatic melanoma.
Another proliferation marker currently under investigation is proliferation cell nuclear antigen (PCNA). PCNA is a polymerase accessory protein that is present throughout the cell cycle, most significantly in the late G1/S phase, and functions as a cofactor of DNA polymerase delta [82]. As a nuclear stain, PCNA has demonstrated the highest staining intensity in metastatic malignant melanoma. Studies have thus suggested that this protein may be correlated with tumor grade [83].
A major problem with the interpretation of melanocytic lesions is the lack of diagnostic reproducibility among dermatopathologists [84]. The battery of stains most frequently used are those that highlight the location and density of melanocytes within the epidermis. Melan-A (MART-1) and MITF are melanocyte specific markers that stain melanocyte cytoplasm and nuclei, respectively [73]. While these markers are extremely helpful in highlighting the architectural features of the melanocytic proliferation, they provide no information regarding the cytology or biological nature of the melanocytes in question. Therefore, a marker which highlights the biological significance of melanocytic proliferations would be helpful.
8. Role of R21 (sAC) immunohistochemistry in melanocytic proliferations
sAC immunohistochemical staining of various benign and malignant melanocytic lesions using the R21 antibody revealed distinct patterns of sAC expression [85]. While all anti-sAC monoclonal antibodies were evaluated R21 had the best efficacy as it relates to melanocytic proliferations.
8.1. Normal melanocytes and benign nevi
Normal intraepidermal melanocytes stained with R21 demonstrated a discrete perinuclear dotlike pattern of staining. Similarly, melanocytes present in benign melanocytic growths such as lentigo simplex, solar lentigo, and chronic photoactivation exhibited the identical pattern of staining as normal melanocytes. Benign congenital and common acquired nevi without morphological features of dysplasia and nevi of special sites also demonstrated this discrete perinuclear dotlike staining pattern. Although rare isolated granular nuclear and nucleolar staining may occasionally be seen, the staining intensities are similar to those of the background keratinocytes. Rare isolated melanocytes with pannuclear staining may be identified in a minority of cases of benign nevi.
8.2. Superficial spreading melanoma
In superficial spreading melanomas, pannuclear staining for R21 is seen in 25–100% of malignant melanocytes [85]. There appears to be increased variability in the types of staining patterns seen within the malignant melanocyte population of any given tumor of this type. Some malignant melanocytes exhibit a broad perinuclear pattern of staining, some malignant melanocytes lack nuclear staining altogether, and others may demonstrate nucleolar staining. Small perinuclear dotlike staining is seen in less than 10% of cases. Melanomas arising within preexisting nevi have been shown to demonstrate pannuclear staining, whereas the benign nevus cells demonstrate perinuclear dotlike staining. Although the presence of pannuclear staining in greater than 25% of melanocytes appears to provide high specificity for establishing a diagnosis of melanoma, larger studies evaluating atypical melanocytic nevi and superficial spreading type melanomas are needed to better assess sensitivity.
8.3. Nodular melanomas
Nodular melanomas exhibit pannuclear staining, but in fewer cells compared with other melanoma subtypes [85]. The remaining cells exhibit either ill-defined “granular” nuclear staining or nucleolar staining. Intense and diffuse cytoplasmic staining of R21 in nodular melanomas appears to be a unique feature. Although diffuse cytoplasmic staining may be seen in other forms of melanoma, the staining in nodular melanoma is both quantitatively and qualitatively greater. Discrete perinuclear dotlike staining is typically lost. Additional larger studies are needed to confirm if this pattern of staining is useful as a diagnostic adjunct in diagnosing these tumors.
8.4. Metastatic melanoma and capsular nevus
Metastatic melanomas with a spindle cell morphology exhibit a pannuclear staining pattern for R21, but lesions with an epithelioid morphology exhibit various patterns of staining including pannuclear, loss of perinuclear dotlike staining, intense diffuse cytoplasmic staining, and no staining [7]. When assessing lymph nodes for melanoma metastasis, it is not uncommon to find small clusters of banal nevomelanocytes within the lymph node capsule. These foci are referred to as capsular nevi. On rare occasion, the distinction of benign nevus from metastatic melanoma may be difficult due to cytologic atypia. These benign capsular nevi exhibit a weak cytoplasmic or perinuclear dotlike pattern of staining with R21 [85].
8.5. Lentiginous malignant melanomas
Lentiginous melanocytic proliferations are another type of melanocytic lesion characterized by proliferating melanocytes along the dermal epidermal junction in a solitary linear fashion of varying densities. Benign lentigo simplex and solar lentigo represent the benign end of the spectrum where the melanocytes are of low density, small in size, and contain inconspicuous nucleoli. In situ mucosal melanoma, acral lentiginous melanoma, and lentigo maligna are characterized by confluent, “back-to-back,” single file growth of melanocytes along the basal layer. These malignant lesions contain melanocytes that exhibit pleomorphism, enlarged nuclei, and prominent nucleoli. In some cases of chronically sun exposed skin, melanocytic hyperplasia along the basal layer may only focally approach confluence and the cytologic features may be abnormal but equivocal for malignancy [86]. These cases that do not fulfill the full criteria for lentigo maligna are referred to as chronic photoactivation of melanocytes and are another frequent source of diagnostic difficulty. Since lentigo maligna melanomas arise in a background of chronic UV exposure, the determination of where melanoma stops and chronic photoactivation begins is extraordinarily difficult even with adjunct melanocyte specific immunohistochemical markers (i.e. Melan-A, HMB45, and MITF). In acral lentiginous and lentigo maligna melanomas a R21 pannuclear staining pattern with greater intensity than the surrounding keratinocytes is seen consistently. Within any given case, the percentage of malignant melanocytes with pannuclear sAC expression is greater than 90% of the total tumor cell population (Fig. 1). In acral lentiginous melanoma, and to a lesser extent lentigo maligna, a superimposed thick broad perinuclear staining pattern may be occasionally seen in random tumor cells, however a discrete perinuclear dotlike pattern without intense nuclear staining is absent. A recent study has investigated the use of R21 immunohistochemcial staining in evaluating margins of lentigo melanoma melanoma [23]. The authors propose that a diluted methodology for R21 immunostaining results in less keratinocyte staining with preserved pannuclear staining of melanoma cells. At this dilution, the perinuclear dotlike pattern is faint or absent. Therefore, the authors propose that the presence or absence of pannuclear staining results in a more simplified interpretation. When assessing lentigo maligna margin they concluded that more than nine R21 pannuclear staining melanocytes within one high-power field and/or a R21 to Melan-A ratio greater than 0.5 are associated with a positive margin. Conversely, five or less R21 pannuclear staining melanocytes per high-power field and/or a R21 to Melan-A ratio less than 0.3 was associated with a negative margin. As a comparison, non-melanoma skin cancer re-excisions showed a faint benign perinuclear dotlike staining pattern within melanocytes, consistent with the notion that pannuclear staining with R21 is a feature of lentigo melanoma cells only [23]. This algorithmic approach with R21 immunostaining was recently applied to the technique of slow Mohs for lentigo maligna melanoma. In this paper the authors compared R21 to MITF for the semi-rapid assessment of lentigo maligna margins and found R21 to be more specific than MITF [87]. Recent work from our group suggests that R21 could be applied to Mohs frozen sections for the rapid surgical clearance of lentigo maligna at the bedside (Drs. Desman and Minkis, pers. comm).
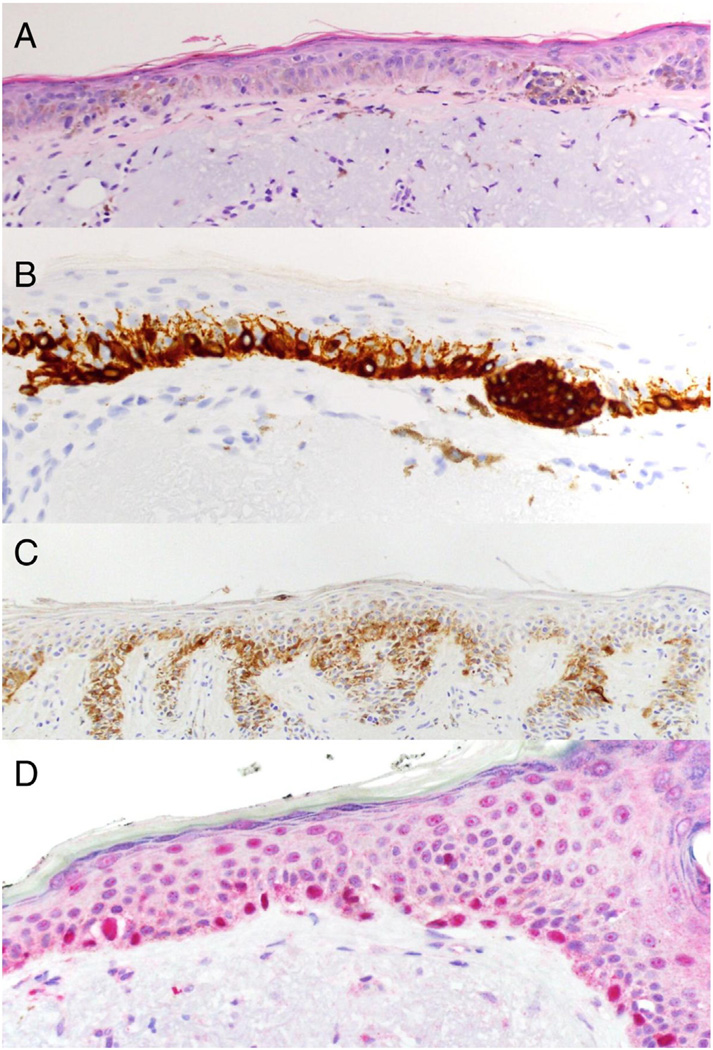
Fig 1.
Comparison of IHC markers in lentigo maligna. A) Hematoxylin and eosin staining of lentigo maligna showing multiple atypical melanocytes along the dermal–epidermal junction and large atypical nests (40×). B) Melan-A in brown stain (40×) and C) HMB45 in brown stain showing a confluent staining of melanocytes (20×). D) R21 (sAC) in red stain with a pannuclear localization (40×). Human tissue samples were formalin fixed and paraffin embedded followed by antigen retrieval and immunohistochemistry as described in [23].
8.6. Melanocytic lesions associated with fibrosis
The histopathologic interpretation of melanocytic lesions within areas of fibrosis can be diagnostically challenging for the dermatopatholgist due to the disruption of the architecture in which the melanocytes colonize the epidermis and dermis [88]. Additionally, the melanocytes overlying various fibrosing dermal proliferations, such as scar beds, can exhibit varying degrees of cytologic atypia presumably attributed to regional growth factor and cytokine milieus. Common causes for these phenomena include chronic irritation/superficial trauma, prior biopsy, and regression. Intraepidermal melanocytes overlying fibrous papules may exhibit an increased density with significant cytologic atypia and thus mimick lentigo maligna melanoma [89]. Lastly, genital nevi with superimposed changes of lichen sclerosus may exhibit severe architectural distortion mimicking melanoma. While immunohistochemical staining for Melan-A may be useful in highlighting the distorted architecture, such as confluent growth and pagetoid ascent, it does not provide any information regarding the biological behavior of these cells. As previously mentioned, HMB45 is positive in junctional and papillary dermal nevus cells as well as stimulated normal melanocytes overlying scar beds and in areas of chronic photoactivation, and is therefore not useful in distinguishing benignancy from malignancy in these situations. In Magro et al. [85]. R21 was examined in recurrent/residual nevi and found to have a preserved perinuclear dotlike staining in the areas where the melanocytes demonstrated enhanced architectural and cytologic atypia in zones of cicatrix. Likewise, in the study by Magro et al. where a diluted methodology for R21 staining was applied to re-excision specimens of reactive lentiginous melanocytic hyperplasia and lentigo maligna, pannuclear staining was not reported in the hyperplasia cases associated with cicatrix but was present in the cases of lentigo maligna [85]. A recently reported case of genital nevus arising in the background of lichen sclerosus in a 7-year-old female presented with markedly atypical architectural and cytologic features, but was confirmed benign after the absence of cytogenetic abnormalities by Fluorescence in situ hybridization (FISH) assay for RREB1 (6p25), MYB (6q23), CCND1 (11q13) genes, and centromere 6. Immunohistochemical staining with R21 revealed a perinuclear dotlike pattern of staining without pannuclear staining [90]. Recently, the expression of HMB45 and R21 were compared in benign lentiginous melanocytic hyperplasias overlying fibrous papules in which varying densities and degrees of cytologic atypia are frequently encountered. While lesions sampled at the base rarely pose diagnostic difficulty, superficially sampled lesions with little dermis can be indistinguishable from lentigo maligna. We now report a study of six cases of fibrous papule with overlying benign lentiginous melanocytic hyperplasia and three cases of lentigo maligna melanoma as a positive control. All cases of lentigo maligna showed confluent lentiginous growth of melanocytes with Melan-A, positive immunoreactivity for HMB45, and a pan-nuclear staining pattern with R21 (Fig. 1a – d). In comparison, all six fibrous papule cases exhibited a lentiginous melanocytic hyperplasia along the basal layer with melanocytes exhibiting an enlarged epithelioid appearance with retraction artifact and enlarged nuclei (Fig. 2a). Characteristic stromal features of fibrous papule were present in the dermis of all cases. The Melan-A stain revealed an increased density of dendritic melanocytes along the dermal epidermal junction with focal areas of confluent growth. In irritated lesions, it was not uncommon to see rare melanocytes above the basal layer (Fig. 2b). All cases stained with HMB45 revealed positivity within the melanocytes (Fig. 2c). The R21 immunostaining revealed absent pannuclear and weak perinuclear dot staining in all cases (Fig. 2d). Therefore, in superficially sampled melanocytic hyperplasias overlying fibrous papule lesions or cicatrix where the histological differential diagnosis includes lentigo maligna, the R21 expression profile is promising as a diagnostic adjunct for differentiating benign from malignant melanocytic hyperplasias.
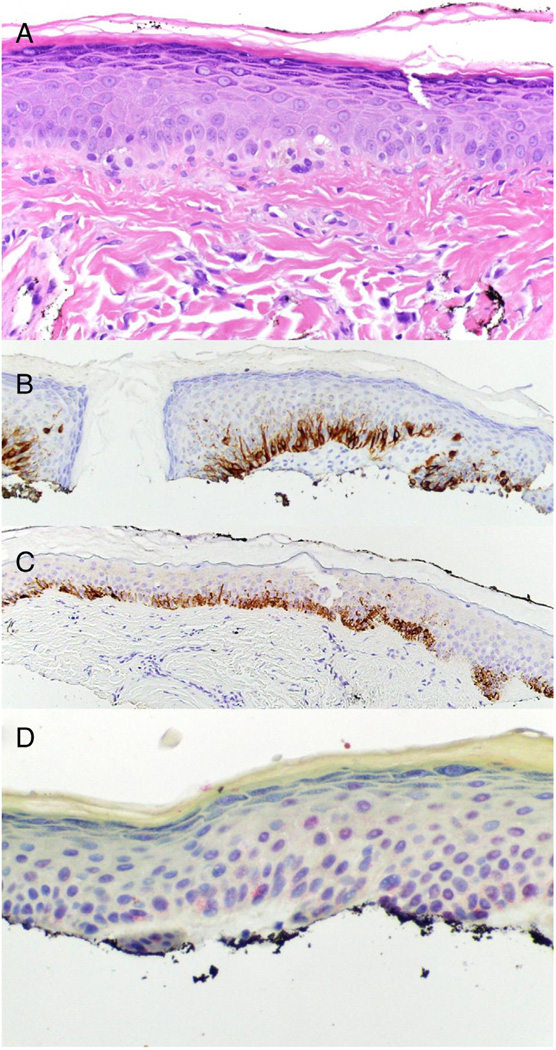
Fig 2.
R21 has a benign staining pattern in melanocytic lesions overlying a cicatrix. A) Hematoxylin and eosin staining of fibrous papule revealing an increased density of enlarged basal layer melanocytes (40×). B) Melan-A, brown stain, reveals increased density of melanocytes approaching confluence (20×). C) HMB45, brown stain, reveals increased density of melanocytes with rare cells above the basal layer in an area of irritation (20×). D) R21 (sAC), red stain, reveals perinuclear dot (benign) staining in melanocytes (40×).
In summary, the R21 antibody has proven a useful adjunct in the assessment of pigmented lesions. Benign melanocytes have a reproducible golgi staining pattern whereas bona fide malignant melanocytes demonstrate a predominance of pan-nuclear staining. The pan-nuclear R21 immunostaining pattern is only present in malignant melanocytes making this pattern highly specific for melanoma. While some subtypes of melanoma have pan-nuclear staining in nearly all the melanocytes (lentiginous growth melanomas), other subtypes (superficial spreading melanoma) may have a limited number of pan-nuclear positive cells. Therefore, depending on the subtype of melanoma, the R21 immunostaining pattern has a variable sensitivity for the diagnosis of melanoma. Because of the stark difference in staining pattern between benign and malignant melanocytes, our group and others are actively investigating whether R21 immunstaining is useful in establishing incipient melanoma.
9. Conclusion
It has become evident that cAMP signaling can occur in multiple distinct microdomains throughout the cell, which are initiated by both tmACs and sAC and are facilitated by multiple effector and regulatory proteins. In diseases such as cancer, the effects of cAMP signaling pathways can sometimes present conflicting outcomes, which most likely result from the presence of multiple cAMP microdomains in those cells. The use of global cAMP activating drugs such as membrane permeable cAMP analogs, forskolin, and pan-specific PDE inhibitors make separating each of these cAMP microdomains difficult. The challenge going forward will be the development of sensitive live cell monitoring techniques and more specific, physiologically relevant tools for changing cAMP level in living cells. Investigation of cAMP signaling in melanoma suggests that there are two sets of cAMP dependent pathways: one that leads to cell growth and one that is inhibitory. sAC is known to change localization when cells transition from a differentiated state to a proliferative state and as detailed in this review, we and others have been able to leverage this observation to improve the diagnostic assessment of melanoma. sAC nuclear localization is a strong indicator of malignant transformation of melanocytes. Furthermore, sAC staining provides a high level of specificity for the diagnosis of melanoma, especially in lentigo maligna melanoma, a unique quality among melanoma IHC markers. In addition, sAC staining has the potential to aid in the rapid assessment of lentiginous growth melanoma margins during MOHs surgery and the difficult assessment of melanocytic lesions overlying cicatrix, a frequent diagnostic conundrums. It is still unknown whether the presence of sAC in the nucleus or the percentage of cells with sAC in the nucleus has any prognostic value for the evaluation of melanoma, and this is the focus of active research. Furthermore, it is imperative to determine the normal function of sAC in different microdomains in benign cells and cancers such as melanoma to determine if sAC represents a novel therapeutic target.
Acknowledgments
This work was supported by the NIH/NCI 1K08 CA 160657-01 (JHZ) and the Albert Einstein School of Medicine Research Fellowship (CW)
Abbreviations
- cAMP
Cyclic adenosine monophosphate
- sAC
Soluble adenylyl cyclase
- tmAC
Transmembrane adenylyl cyclase
- PDE
Phosphodiesterase
- PKA
Protein kinase A
- AKAP
A kinase anchoring protein
- EPAC
Exchange protein activated by cAMP
- GEF
Guanine nucleotide exchange factor
- GAP
GTPase–activating proteins
- HMB
Human melanoma black
- MITF
Microphthalmia transcription factor
Footnotes
This article is part of a Special Issue entitled: The role of soluble adenylyl cyclase in health and disease.
Author contributions
CW, GD, and JHZ wrote the manuscript. JHZ edited the manuscript.
References
- 1.Sabbatini ME, Gorelick F, Glaser S. Adenylyl cyclases in the digestive system. Cell. Signal. 2014;26:1173–1181. doi: 10.1016/j.cellsig.2014.01.033. http://dx.doi.org/10.1016/j.cellsig.2014.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahman N, Buck J, Levin LR. pH sensing via bicarbonate-regulated "soluble" adenylyl cyclase (sAC) Front. Physiol. 2013;4:343. doi: 10.3389/fphys.2013.00343. http://dx.doi.org/10.3389/fphys.2013.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zippin JH, et al. Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains. FASEB J. 2003;17:82–84. doi: 10.1096/fj.02-0598fje. http://dx.doi.org/10.1096/fj.02-0598fje. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, et al. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289:625–628. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- 5.Litvin TN, Kamenetsky M, Zarifyan A, Buck J, Levin LR. Kinetic properties of “soluble” adenylyl cyclase. Synergism between calcium and bicarbonate. J. Biol. Chem. 2003;278:15922–15926. doi: 10.1074/jbc.M212475200. http://dx.doi.org/10.1074/jbc.M212475200. [DOI] [PubMed] [Google Scholar]
- 6.Pastor-Soler N, et al. Bicarbonate-regulated adenylyl cyclase (sAC) is a sensor that regulates pH-dependent V-ATPase recycling. J. Biol. Chem. 2003;278:49523–49529. doi: 10.1074/jbc.M309543200. http://dx.doi.org/10.1074/jbc.M309543200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Awad JA, Johnson RA, Jakobs KH, Schultz G. Interactions of forskolin and adenylate cyclase. Effects on substrate kinetics and protection against inactivation by heat and N-ethylmaleimide. J. Biol. Chem. 1983;258:2960–2965. [PubMed] [Google Scholar]
- 8.Zippin JH, et al. CO2/HCO3(−)- and calcium-regulated soluble adenylyl cyclase as a physiological ATP sensor. J. Biol. Chem. 2013;288:33283–33291. doi: 10.1074/jbc.M113.510073. http://dx.doi.org/10.1074/jbc.M113.510073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramos LS, Zippin JH, Kamenetsky M, Buck J, Levin LR. Glucose and GLP-1 stimulate cAMP production via distinct adenylyl cyclases in INS-1E insulinoma cells. J. Gen. Physiol. 2008;132:329–338. doi: 10.1085/jgp.200810044. http://dx.doi.org/10.1085/jgp.200810044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaloupka JA, Bullock SA, Iourgenko V, Levin LR, Buck J. Autoinhibitory regulation of soluble adenylyl cyclase. Mol. Reprod. Dev. 2006;73:361–368. doi: 10.1002/mrd.20409. http://dx.doi.org/10.1002/mrd.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Middelhaufe S, Leipelt M, Levin LR, Buck J, Steegborn C. Identification of a haem domain in human soluble adenylate cyclase. Biosci. Rep. 2012;32:491–499. doi: 10.1042/BSR20120051. http://dx.doi.org/10.1042/BSR20120051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinberg SF, Brunton LL. Compartmentation of G protein-coupled signaling pathways in cardiac myocytes. Annu. Rev. Pharmacol. Toxicol. 2001;41:751–773. doi: 10.1146/annurev.pharmtox.41.1.751. http://dx.doi.org/10.1146/annurev.pharmtox.41.1.751. [DOI] [PubMed] [Google Scholar]
- 13.Papa S, Sardanelli AM, Scacco S, Technikova-Dobrova Z. cAMP-dependent protein kinase and phosphoproteins in mammalian mitochondria. An extension of the cAMP-mediated intracellular signal transduction. FEBS Lett. 1999;444:245–249. doi: 10.1016/s0014-5793(99)00070-8. [DOI] [PubMed] [Google Scholar]
- 14.Kawasaki H, et al. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- 15.Niimura M, et al. Critical role of the N-terminal cyclic AMP-binding domain of Epac2 in its subcellular localization and function. J. Cell. Physiol. 2009;219:652–658. doi: 10.1002/jcp.21709. http://dx.doi.org/10.1002/jcp.21709. [DOI] [PubMed] [Google Scholar]
- 16.de Rooij J, et al. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. http://dx.doi.org/10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt M, Dekker FJ, Maarsingh H. Exchange protein directly activated by cAMP (epac): a multidomain cAMP mediator in the regulation of diverse biological functions. Pharmacol. Rev. 2013;65:670–709. doi: 10.1124/pr.110.003707. http://dx.doi.org/10.1124/pr.110.003707. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, et al. The RAP1 guanine nucleotide exchange factor Epac2 couples cyclic AMP and Ras signals at the plasma membrane. J. Biol. Chem. 2006;281:2506–2514. doi: 10.1074/jbc.M508165200. http://dx.doi.org/10.1074/jbc.M508165200. [DOI] [PubMed] [Google Scholar]
- 19.Huston E, et al. EPAC and PKA allow cAMP dual control over DNA-PK nuclear translocation. Proc. Natl. Acad. Sci. U. S. A. 2008;105:12791–12796. doi: 10.1073/pnas.0805167105. http://dx.doi.org/10.1073/pnas.0805167105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metrich M, et al. Epac activation induces histone deacetylase nuclear export via a Ras-dependent signalling pathway. Cell. Signal. 2010;22:1459–1468. doi: 10.1016/j.cellsig.2010.05.014. http://dx.doi.org/10.1016/j.cellsig.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Sample V, et al. Regulation of nuclear PKA revealed by spatiotemporal manipulation of cyclic AMP. Nat Chem Biol. 2012;8:375–382. doi: 10.1038/nchembio.799. http://dx.doi.org/10.1038/nchembio.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zippin JH, Chadwick PA, Levin LR, Buck J, Magro CM. Soluble adenylyl cyclase defines a nuclear cAMP microdomain in keratinocyte hyperproliferative skin diseases. J. Invest Dermatol. 2010;130:1279–1287. doi: 10.1038/jid.2009.440. http://dx.doi.org/10.1038/jid.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magro CM, Yang SE, Zippin JH, Zembowicz A. Expression of soluble adenylyl cyclase in lentigo maligna: use of immunohistochemistry with anti-soluble adenylyl cyclase antibody (R21) in diagnosis of lentigo maligna and assessment of margins. Arch. Pathol. Lab. Med. 2012;136:1558–1564. doi: 10.5858/arpa.2011-0617-OA. http://dx.doi.org/10.5858/arpa.2011-0617-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Francis SH, Corbin JD. Structure and function of cyclic nucleohde-dependent protein kinases. Annu. Rev. Physiol. 1994;56:237–272. doi: 10.1146/annurev.ph.56.030194.001321. http://dx.doi.org/10.1146/annurev.ph.56.030194.001321. [DOI] [PubMed] [Google Scholar]
- 25.Scott JD. Cyclic nucleotide-dependent protein kinases. Pharmacol. Ther. 1991;50:123–145. doi: 10.1016/0163-7258(91)90075-w. [DOI] [PubMed] [Google Scholar]
- 26.Cummings DE, et al. Genetically lean mice result from targeted disruption of the RII beta subunit of protein kinase A. Nature. 1996;382:622–626. doi: 10.1038/382622a0. http://dx.doi.org/10.1038/382622a0. [DOI] [PubMed] [Google Scholar]
- 27.Taylor SS, et al. Dynamics of signaling by PKA. Biochim. Biophys. Acta. 2005;1754:25–37. doi: 10.1016/j.bbapap.2005.08.024. http://dx.doi.org/10.1016/j.bbapap.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 28.Zippin JH, et al. Bicarbonate-responsive “soluble” adenylyl cyclase defines a nuclear cAMP microdomain. J. Cell Biol. 2004;164:527–534. doi: 10.1083/jcb.200311119. http://dx.doi.org/10.1083/jcb.200311119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terrin A, et al. PKA and PDE4D3 anchoring to AKAP9 provides distinct regulation of cAMP signals at the centrosome. J. Cell Biol. 2012;198:607–621. doi: 10.1083/jcb.201201059. http://dx.doi.org/10.1083/jcb.201201059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welch EJ, Jones BW, Scott JD. Networking with AKAPs: context-dependent regulation of anchored enzymes. Mol. Interv. 2010;10:86–97. doi: 10.1124/mi.10.2.6. http://dx.doi.org/10.1124/mi.10.2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carr DW, et al. Interaction of the regulatory subunit (RII) of cAMP-dependent protein kinase with RII-anchoring proteins occurs through an amphipathic helix binding motif. J. Biol. Chem. 1991;266:14188–14192. [PubMed] [Google Scholar]
- 32.Alto NM, et al. Bioinformatic design of A-kinase anchoring protein-in silico: a potent and selective peptide antagonist of type II protein kinase A anchoring. Proc. Natl. Acad. Sci. U. S. A. 2003;100:4445–4450. doi: 10.1073/pnas.0330734100. http://dx.doi.org/10.1073/pnas.0330734100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kapiloff MS, Rigatti M, Dodge-Kafka KL. Architectural and functional roles of A kinase-anchoring proteins in cAMP microdomains. J. Gen. Physiol. 2014;143:9–15. doi: 10.1085/jgp.201311020. http://dx.doi.org/10.1085/jgp.201311020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Esseltine JL, Scott JD. AKAP signaling complexes: pointing towards the next generation of therapeutic targets? Trends Pharmacol. Sci. 2013;34:648–655. doi: 10.1016/j.tips.2013.10.005. http://dx.doi.org/10.1016/j.tips.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coghlan VM, Langeberg LK, Fernandez A, Lamb NJ, Scott JD. Cloning and charac- 946 terization of AKAP 95, a nuclear protein that associates with the regulatory subunit of type II cAMP-dependent protein kinase. J. Biol. Chem. 1994;269:7658–7665. [PubMed] [Google Scholar]
- 36.Arsenijevic T, Degraef C, Dumont JE, Roger PP, Pirson I. G1/S Cyclins interact with regulatory subunit of PKA via A-kinase anchoring protein, AKAP95. Cell Cycle. 2006;5:1217–1222. doi: 10.4161/cc.5.11.2802. [DOI] [PubMed] [Google Scholar]
- 37.Collas P, Le Guellec K, Tasken K. The A-kinase-anchoring protein AKAP95 is a multivalent protein with a key role in chromatin condensation at mitosis. J. Cell Biol. 1999;147:1167–1180. doi: 10.1083/jcb.147.6.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown RL, August SL, Williams CJ, Moss SB. AKAP7gamma is a nuclear RI-binding AKAP. Biochem Biophys. Res. Commun. 2003;306:394–401. doi: 10.1016/s0006-291x(03)00982-3. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Q, Carr DW, Lerea KM, Scott JD, Newman SA. Nuclear localization of type II cAMP-dependent protein kinase during limb cartilage differentiation is associated with a novel developmentally regulated A-kinase anchoring protein. Dev. Biol. 1996;176:51–61. doi: 10.1006/dbio.1996.9995. http://dx.doi.org/10.1006/dbio.1996.9995. [DOI] [PubMed] [Google Scholar]
- 40.Feng Q, et al. Two domains are critical for the nuclear localization of soluble adenylyl cyclase. Biochimie. 2006;88:319–328. doi: 10.1016/j.biochi.2005.09.003. http://dx.doi.org/10.1016/j.biochi.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 41.Yang JH, Polanowska-Grabowska RK, Smith JS, Shields CWt, Saucerman JJ. PKA catalytic subunit compartmentation regulates contractile and hypertrophic responses to beta-adrenergic signaling. J. Mol. Cell. Cardiol. 2014;66:83–93. doi: 10.1016/j.yjmcc.2013.11.001. http://dx.doi.org/10.1016/j.yjmcc2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diviani D, Langeberg LK, Doxsey SJ, Scott JD. Pericentral anchors protein kinase A at the centrosome through a newly identified RII-binding domain. Curr. Biol. 2000;10:417–420. doi: 10.1016/s0960-9822(00)00422-x. [DOI] [PubMed] [Google Scholar]
- 43.Means CK, et al. An entirely specific type I A-kinase anchoring protein that can sequester two molecules of protein kinase A at mitochondria. Proc. Natl. Acad. Sri. U. S. A. 2011;108:E1227–E1235. doi: 10.1073/pnas.1107182108. http://dx.doi.org/10.1073/pnas.1107182108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alto NM, Soderling J, Scott JD. Rab32 is an A-kinase anchoring protein and participates in mitochondrial dynamics. J. Cell Biol. 2002;158:659–668. doi: 10.1083/jcb.200204081. http://dx.doi.org/10.1083/jcb.200204081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dyson MT, et al. Mitochondrial A-kinase anchoring protein 121 binds type II protein kinase A and enhances steroidogenic acute regulatory protein-mediated steroidogenesis in MA-10 mouse leydig tumor cells. Biol. Reprod. 2008;78:267–277. doi: 10.1095/biolreprod.107.064238. http://dx.doi.org/10.1095/biolreprod.107.064238. [DOI] [PubMed] [Google Scholar]
- 46.Sardanelli AM, et al. Occurrence of A-kinase anchor protein and associated cAMP-dependent protein kinase in the inner compartment of mammalian mitochondria. FEBS Lett. 2006;580:5690–5696. doi: 10.1016/j.febslet.2006.09.020. http://dx.doi.org/10.1016/j.febslet2006.09.020. [DOI] [PubMed] [Google Scholar]
- 47.Abrenica B, AlShaaban M, Czubryt MP. The A-kinase anchor protein AKAP121 is a negative regulator of cardiomyocyte hypertrophy. J. Mol. Cell. Cardiol. 2009;46:674–681. doi: 10.1016/j.yjmcc.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 48.Wu KY, et al. Soluble adenylyl cyclase is required for netrin-1 signaling in nerve growth cones. Nat. Neurosci. 2006;9:1257–1264. doi: 10.1038/nn1767. http://dx.doi.org/10.1038/nn1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dumaz N, et al. In melanoma, RAS mutations are accompanied by switching signaling from BRAF to CRAF and disrupted cyclic AMP signaling. Cancer Res. 2006;66:9483–9491. doi: 10.1158/0008-5472.CAN-05-4227. http://dx.doi.org/10.1158/0008-5472.CAN-05-4227. [DOI] [PubMed] [Google Scholar]
- 50.Dumaz N. Mechanism of RAF isoform switching induced by oncogenic RAS in melanoma. Small GTPases. 2011;2:289–292. doi: 10.4161/sgtp.2.5.17814. http://dx.doi.org/10.4161/sgtp.2.5.17814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marquette A, Andre J, Bagot M, Bensussan A, Dumaz N. ERK and PDE4 cooperate to induce RAF isoform switching in melanoma. Nat. Struct. Mol. Biol. 2011;18:584–591. doi: 10.1038/nsmb.2022. http://dx.doi.org/10.1038/nsrnb.2022. [DOI] [PubMed] [Google Scholar]
- 52.Rigel DS, Russak J, Friedman R. The evolution of melanoma diagnosis: 25 years beyond the ABCDs. CA Cancer J. Clin. 2010;60:301–316. doi: 10.3322/caac.20074. http://dx.doi.org/10.3322/caac20074. [DOI] [PubMed] [Google Scholar]
- 53.Barnhill RL. Textbook of dermatopathology. 3rd edn. McGraw-Hill; 2010. [Google Scholar]
- 54.Farber MJ, Heilman ER, Friedman RJ. Dysplastic nevi. Dermatol. Clin. 2012;30:389–404. doi: 10.1016/j.det.2012.04.004. http://dx.doi.org/10.1016/j.det.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 55.Moore BW. A soluble protein characteristic of the nervous system. Biochem. Biophys. Res. Commun. 1965;19:739–744. doi: 10.1016/0006-291x(65)90320-7. [DOI] [PubMed] [Google Scholar]
- 56.Fernando SS, Johnson S, Bate J. Immunohistochemical analysis of cutaneous malignant melanoma: comparison of S-100 protein, HMB-45 monoclonal antibody and NKI/C3 monoclonal antibody. Pathology. 1994;26:16–19. doi: 10.1080/00313029400169021. [DOI] [PubMed] [Google Scholar]
- 57.Santamaria-Kisiel L, Rintala-Dempsey AC, Shaw GS. Calcium-dependent and -independent interactions of the S100 protein family. Biochem. J. 2006;396:201–214. doi: 10.1042/BJ20060195. http://dx.doi.org/10.1042/BJ20060195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harpio R, Einarsson R. S100 proteins as cancer biomarkers with focus on S100B in malignant melanoma. Clin. Biochem. 2004;37:512–518. doi: 10.1016/j.clinbiochem.2004.05.012. http://dx.doi.org/10.1016/j.clinbiochem.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 59.Prieto VG, Shea CR. Immunohistochemistry of melanocytic proliferations. Arch. Pathol. Lab. Med. 2011;135:853–859. doi: 10.5858/2009-0717-RAR.1. http://dx.doi.org/10.1043/2009-0717-RAR.1. [DOI] [PubMed] [Google Scholar]
- 60.Lens MB, Newton-Bishop JA, Boon AP. Desmoplastic malignant melanoma: a systematic review. Br. J. Dermatol. 2005;152:673–678. doi: 10.1111/j.1365-2133.2005.06462.x. http://dx.doi.org/10.1111/j.1365-2133.2005.06462.x. [DOI] [PubMed] [Google Scholar]
- 61.Orchard GE. Comparison of immunohistochemical labelling of melanocyte differentiation antibodies melan-A, tyrosinase and HMB 45 with NKIC3 and S100 protein in the evaluation of benign naevi and malignant melanoma. Histochem. J. 2000;32:475–481. doi: 10.1023/a:1004192232357. [DOI] [PubMed] [Google Scholar]
- 62.Sheffield MV, et al. Comparison of five antibodies as markers in the diagnosis of melanoma in cytologic preparations. Am. J. Clin. Pathol. 2002;118:930–936. doi: 10.1309/EWK9-LUPR-6BC5-1GXV. http://dx.doi.org/10.1309/EWK9-LUPR-6BC5-1GXV. [DOI] [PubMed] [Google Scholar]
- 63.Jungbluth AA, et al. A103: an anti-melan-a monoclonal antibody for the detection of malignant melanoma in paraffin-embedded tissues. Am. J. Surg. Pathol. 1998;22:595–602. doi: 10.1097/00000478-199805000-00011. [DOI] [PubMed] [Google Scholar]
- 64.Chen YT, et al. Serological analysis of Melan-A(MART-1), a melanocyte-specific protein homogeneously expressed in human melanomas. Proc. Natl. Acad. Sci. U. S.A. 1996;93:5915–5919. doi: 10.1073/pnas.93.12.5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim J, Taube JM, McCalmont TH, Glusac EJ. Quantitative comparison of MiTF, Melan-A, HMB-45 and Mel-5 in solar lentigines and melanoma in situ. J. Cutan. Pathol. 2011;38:775–779. doi: 10.1111/j.1600-0560.2011.01763.x. http://dx.doi.org/10.1111/j.1600-0560.2011.01763.x. [DOI] [PubMed] [Google Scholar]
- 66.Skelton III HG, Smith KJ, Barrett TL, Lupton GP, Graham JH. HMB-15 staining in benign and malignant melanocytic lesions. A reflection of cellular activation. Am. J. Dermatopathol. 1991;13:543–550. doi: 10.1097/00000372-199113060-00004. [DOI] [PubMed] [Google Scholar]
- 67.Ordonez NG, Ji XL, Hickey RC. Comparison of HMB-45 monoclonal antibody and S-100 protein in the immunohistochemical diagnosis of melanoma. Am. J. Clin. Pathol. 1988;90:385–390. doi: 10.1093/ajcp/90.4.385. [DOI] [PubMed] [Google Scholar]
- 68.Wick MR, Swanson PE, Rocamora A. Recognition of malignant melanoma by monoclonal antibody HMB-45. An immunohistochemical study of 200 paraffin-embedded cutaneous tumors. J. Cutan. Pathol. 1988;15:201–207. doi: 10.1111/j.1600-0560.1988.tb00544.x. [DOI] [PubMed] [Google Scholar]
- 69.Levy C, Fisher DE. Dual roles of lineage restricted transcription factors: the case of MITF in melanocytes. Transcription. 2011;2:19–22. doi: 10.4161/trns.2.1.13650. http://dx.doi.org/10.4161/trns.2.1.13650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Busam KJ, et al. Immunohistochemical analysis of novel monoclonal antibody PNL2 and comparison with other melanocyte differentiation markers. Am. J. Surg. Pathol. 2005;29:400–406. doi: 10.1097/01.pas.0000152137.81771.5b. [DOI] [PubMed] [Google Scholar]
- 71.Aung PP, et al. KBA62 and PNL2: 2 new melanoma markers-immunohistochemical analysis of 1563 tumors including metastatic, desmoplastic, and mucosal melanomas and their mimics. Am. J. Surg. Pathol. 2012;36:265–272. doi: 10.1097/PAS.0b013e31823651cb. http://dx.doi.org/10.1097/PAS.0b013e31823651cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kawakami Y, et al. Production of recombinant MART-1 proteins and specific antiMART-1 polyclonal and monoclonal antibodies: use in the characterization of the human melanoma antigen MART-1. J. Immunol. Methods. 1997;202:13–25. doi: 10.1016/s0022-1759(96)00211-6. [DOI] [PubMed] [Google Scholar]
- 73.Ordonez NG. Value of melanocytic-associated immunohistochemical markers in the diagnosis of malignant melanoma: a review and update. Hum. Pathol. 2014;45:191–205. doi: 10.1016/j.humpath.2013.02.007. http://dx.doi.org/10.1016/j.humpath.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 74.Miettinen M, et al. Microphthalmia transcription factor in the immunohistochemical diagnosis of metastatic melanoma: comparison with four other melanoma markers. Am J. Surg. Pathol. 2001;25:205–211. doi: 10.1097/00000478-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 75.Busam KJ. Jungbluth, Melan-A, a new melanocytic differentiation marker. Adv. Anat Pathol. 1999;6:12–18. doi: 10.1097/00125480-199901000-00002. [DOI] [PubMed] [Google Scholar]
- 76.King R, Googe PB, Weilbaecher KN, Mihm MC, Jr, Fisher DE. Microphthalmia transcription factor expression in cutaneous benign, malignant melanocytic, and nonmelanocytic tumors. Am. J. Surg. Pathol. 2001;25:51–57. doi: 10.1097/00000478-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 77.Pages C, et al. KBA62: a useful marker for primary and metastatic melanomas. Hum Pathol. 2008;39:1136–1142. doi: 10.1016/j.humpath.2007.12.006. http://dx.doi.org/10.1016/j.humpath.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 78.Smolle J, Soyer HP, Kerl H. Proliferative activity of cutaneous melanocytic tumors defined by Ki-67 monoclonal antibody. A quantitative immunohistochemical study. Am J. Dermatopathol. 1989;11:301–307. doi: 10.1097/00000372-198908000-00002. [DOI] [PubMed] [Google Scholar]
- 79.Soyer HP. Ki 67 immunostaining in melanocytic skin tumors. Correlation with histologic parameters. J. Cutan. Pathol. 1991;18:264–272. doi: 10.1111/j.1600-0560.1991.tb01234.x. [DOI] [PubMed] [Google Scholar]
- 80.Ramsay JA, From L, Iscoe NA, Kahn HJ. MIB-1 proliferative activity is a significant prognostic factor in primary thick cutaneous melanomas. J. Invest. Dermatol. 1995;105:22–26. doi: 10.1111/1523-1747.ep12312431. [DOI] [PubMed] [Google Scholar]
- 81.Hazan C, et al. Evaluation of the proliferation marker MIB-1 in the prognosis of cutaneous malignant melanoma. Cancer. 2002;95:634–640. doi: 10.1002/cncr.10685. http://dx.doi.org/10.1002/cncr.10685. [DOI] [PubMed] [Google Scholar]
- 82.Takahashi H, Strutton GM, Parsons PG. Determination of proliferating fractions in malignant melanomas by anti-PCNA/cyclin monoclonal antibody. Histopathology. 1991;18:221–227. doi: 10.1111/j.1365-2559.1991.tb00829.x. [DOI] [PubMed] [Google Scholar]
- 83.Rieger E, et al. Comparison of proliferative activity as assessed by proliferating cell nuclear antigen (PCNA) and Ki-67 monoclonal antibodies in melanocytic skin lesions. A quantitative immunohistochemical study. J. Cutan. Pathol. 1993;20:229–236. doi: 10.1111/j.1600-0560.1993.tb00648.x. [DOI] [PubMed] [Google Scholar]
- 84.Eriksson H, et al. Interobserver variability of histopathological prognostic parameters in cutaneous malignant melanoma: impact on patient management. Acta Derm. Venereol. 2013;93:411–416. doi: 10.2340/00015555-1517. http://dx.doi.org/10.2340/00015555-1517. [DOI] [PubMed] [Google Scholar]
- 85.Magro CM, Crowson AN, Desman G, Zippin JH. Soluble adenylyl cyclase antibody profile as a diagnostic adjunct in the assessment of pigmented lesions. Arch. Dermatol. 2012;148:335–344. doi: 10.1001/archdermatol.2011.338. http://dx.doi.org/10.1001/archdermatol.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kallini JR, Jain SK, Khachemoune A. Lentigo maligna: review of salient characteristics and management. Am. J. Clin. Dermatol. 2013;14:473–480. doi: 10.1007/s40257-013-0044-6. http://dx.doi.org/10.1007/S40257-013-0044-6. [DOI] [PubMed] [Google Scholar]
- 87.Solky AC, Zembowicz A. Soluble adenylyl cyclase antibody (R21) as a diagnostic adjunct in the evaluation of lentigo maligna margins during slow Mohs surgery. Am J. Dermatopathol. 2014 doi: 10.1097/DAD.0000000000000074. http://dx.doi.org/10.1097/DAD.0000000000000074. [DOI] [PubMed]
- 88.Fox JC, Reed JA, Shea CR. The recurrent nevus phenomenon: a history of challenge, controversy, and discovery. Arch. Pathol. Lab. Med. 2011;135:842–846. doi: 10.5858/2010-0429-RAR.1. http://dx.doi.org/10.1043/2010-0429-RAR.1. [DOI] [PubMed] [Google Scholar]
- 89.Reed RJ. Fibrous papule of the face. Melanocytic angiofibroma. Am. J. Dermatopathol. 1979;1:343–344. doi: 10.1097/00000372-197900140-00009. [DOI] [PubMed] [Google Scholar]
- 90.Pinto A, McLaren SH, Poppas DP, Magro CM. Genital melanocytic nevus arising in a background of lichen sclerosus in a 7-year-old female: the diagnostic pitfall with malignant melanoma. A literature review. Am. J. Dermatopathol. 2012;34:838–843. doi: 10.1097/DAD.0b013e31825d79b3. http://dx.doi.org/10.1097/DAD.0b013e31825d79b3. [DOI] [PubMed] [Google Scholar]