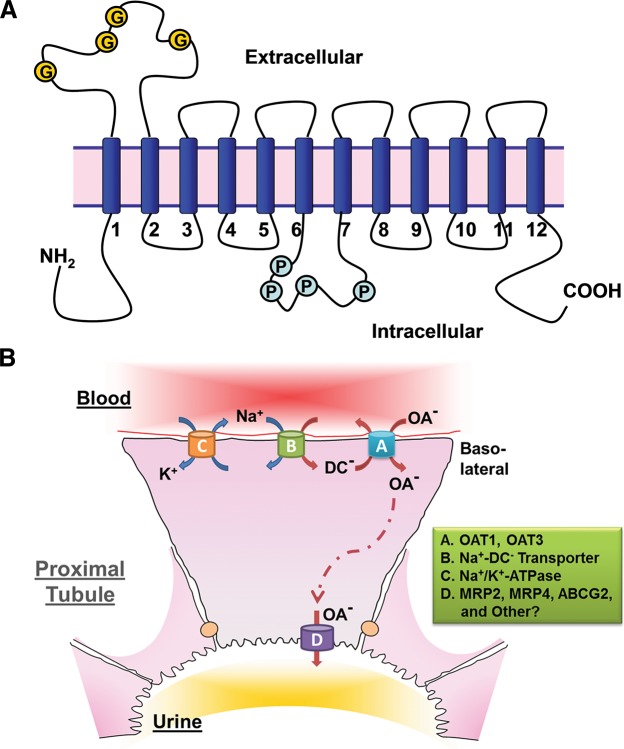
FIGURE 1.
OAT structure and the mechanism of OAT-mediated uptake and transport of organic anions. A: illustration of the predicted topology of organic anion transporters. Two pairs of 6-transmembrane domains are connected by a large intracellular loop and both NH2 and COOH termini are intracellular (G, glycosylation sites; P, PKC phosphorylation sites). B: a renal proximal tubule cell is depicted as a prototypical epithelial cell to illustrate the Oat-mediated uptake and transcellular movement of organic anionic substrates (OA−) from the blood to the urine. Oat1 and Oat3 (A), localized to the basolateral membrane of the proximal tubule cell, transport OA− across the basolateral membrane and into the cell through the exchange of dicarboxylates (DC−). As a secondary active membrane transporter system (76), the Oat-mediated entry of OA− is linked to the transmembrane electrochemical potential of dicarboxylates generated by their movement against a concentration gradient and intracellular accumulation maintained through the action of the Na+/dicarboxylate cotransporter (B). Thus the energy driving this ”tertiary“ mechanism is the ATP consumed by the Na+-K+-ATPase in generating the sodium gradient (C). OA− exit into the urinary luminal space (D) is via transporters found on the apical membrane. [Modified from Eraly et al. (69), with permission from ASPET.]