Fig. 4.
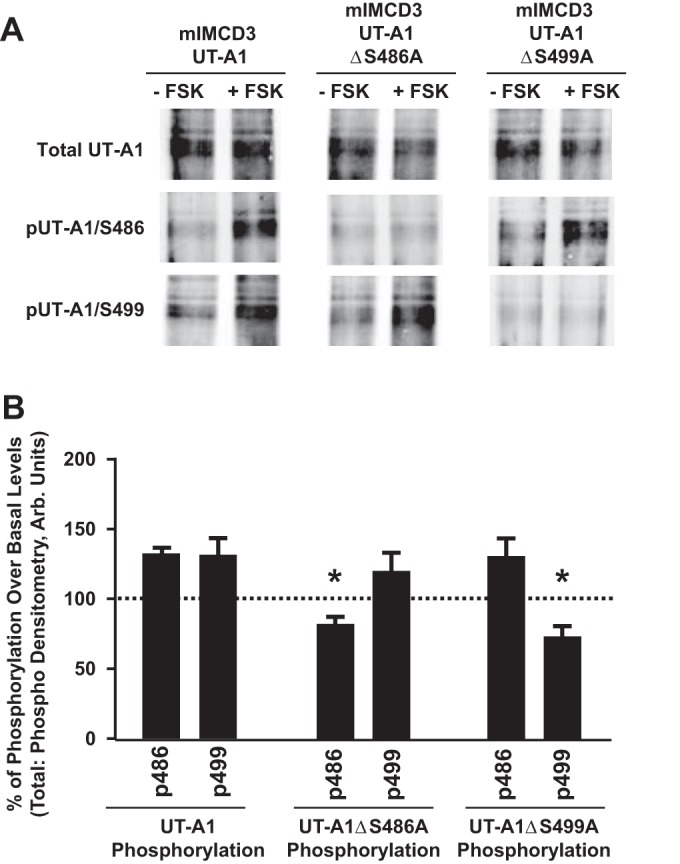
Elevated cAMP stimulates independent phosphorylation of UT-A1 residues S486 and S499. A: mIMCD3 cells were transfected with 1) unaltered UT-A1 (mIMCD3-UT-A1), 2) UT-A1 containing a site-directed mutation at S486 (mIMCD3-UT-A1ΔS486A), or 3) UT-A1 containing a site-directed mutation at S499 (mIMCD3-UT-A1ΔS499A). Thirty-six hours after transfection, cells were incubated with either vehicle (−FSK) or FSK (+FSK; 10 μM) for 20 min at 37°C. After treatment, cells were lysed in RIPA buffer containing protease and phosphatase inhibitors. UT-A1 was enriched in all samples by performing immunoprecipitation with UT-A1 antibody. Shown are representative Western blots probed with UT-A1 antibody (top), the phospho-specific antibody for site S486 (pUT-A1/S486; middle), or the phospho-specific antibody for site S499 (pUT-A1/S499; bottom). B: amount of detected phosphorylation relative to untreated samples, which were normalized to 100%. Values are means ± SE; n = 3. *P < 0.05 (significant difference).
