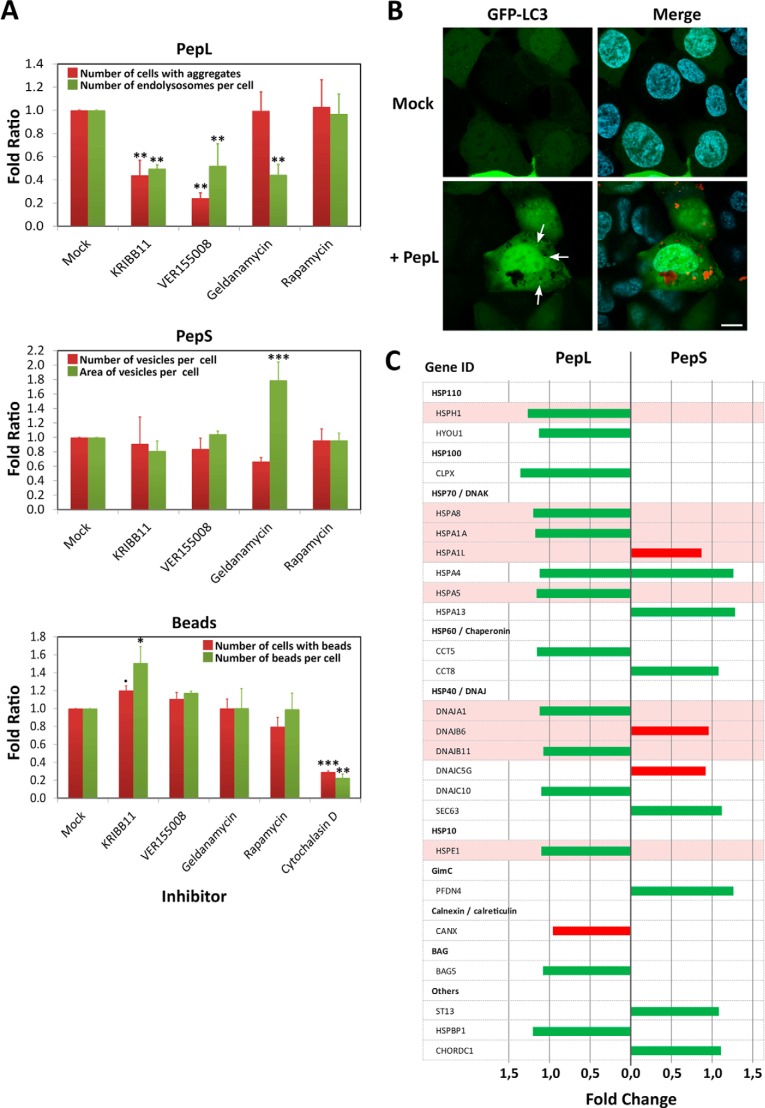
FIGURE 7.
Role of the protein quality control system in the internalization of aggregating peptides. A, selective chemical inhibition of various agents of the protein quality control system. HEK-293 cells were incubated in medium containing 5 μm concentrations of peptides PepL-DyLight 488 or PepS-DyLight 488 or in a 1% suspension of polystyrene microspheres (3-μm diameter Fluoresbrite microparticles, Polysciences, Inc.) in the absence (mock) or presence of the following inhibitors: 10 μm KRIBB11, 40 μm VER155008, 10 μm geldanamycin, 100 nm rapamycin, and 1 μm cytochalasin D. Different quantifications of size and number of the different vesicle types and beads were performed by high content analysis in vivo after 24 h of incubation. Error bars, S.D. of three independent experiments performed in duplicate. Statistical significance is indicated as in Fig. 2C: α ≤ 0.05 (●); α ≤ 0.01 (*); α ≤ 0.001 (**); α = 0 (***). B, LC3 localization. HEK-293 cells expressing a fluorescent fusion protein GFP-LC3 (green) were incubated in medium containing 5 μm PepL-DyLight 550 (red) and analyzed by in vivo confocal microscopy. Images were captured after an overnight incubation. C, expression levels of several representative members of the quality control system after incubation with aggregating peptides. HEK-293 cells were incubated for 24 h in medium containing 5 μm PepL or PepS. Cells were then lysed, and total mRNAs were extracted, purified, and hybridized in an expression profile microarray (Affymetrix Human PrimeView). Up-regulated genes (green bars) and down-regulated genes (red bars) are indicated for each of the peptides. Expression levels are the average of three independent experimental replicates and are represented as the -fold change with respect to untreated controls.