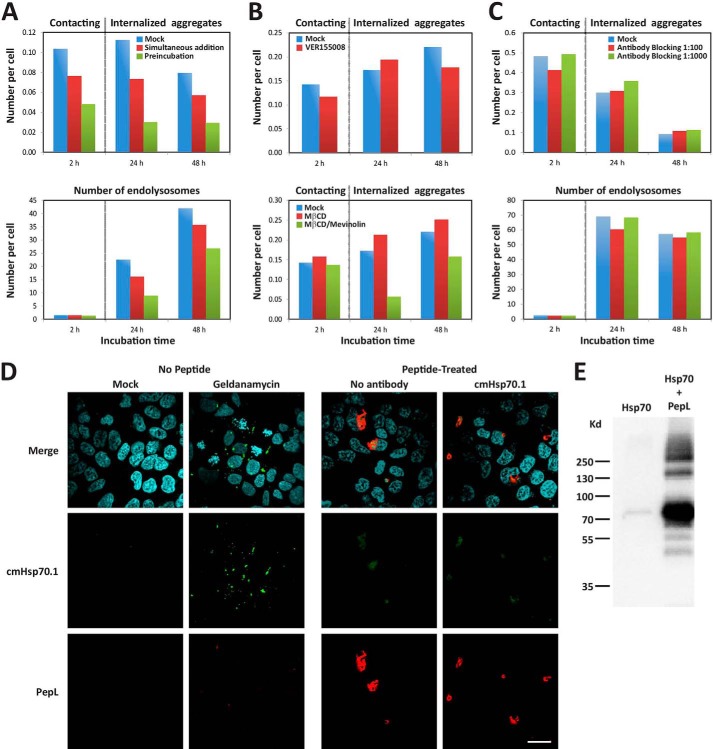
FIGURE 8.
Role of Hsp70 in the internalization of PepL aggregates. A, extracellular addition of Hsp70 protein. A mixed solution of 6 μm PepL and 1.2 μm Hsp70 in PBS was incubated at 37 °C for 1 h and then added to the culture medium of HEK-293 cells at 90% confluence to a final concentration of 2 μm PepL and 400 nm Hsp70 (green bars, Preincubation). Alternatively, a PepL/Hsp70 solution at the same concentration was added to cells without any previous incubation (red bars, Simultaneous addition). As a negative control, a solution containing only 6 μm PepL was added to the cell culture medium (blue bars, Mock). To measure the amount of peptide attached to the cell membranes, the solution containing the peptide was removed after 1 h of incubation, and cells were washed twice with complete medium. The number of aggregates that remained attached to cell membranes was then quantified by high content analysis (2 h time point). 24 and 48 h after peptide addition, the number of internalized aggregates (top) and endolysosomes (bottom) was also quantified by high content analysis. A dotted vertical gray line separates the time points where extracellular aggregates were quantified from time points showing intracellular aggregates. B, effect of Hsp70 inhibition and cholesterol depletion on aggregate membrane attachment. HEK-293 cells were incubated in medium containing 5 μm PepL-DyLight 488 in the absence (mock) or presence of the indicated inhibitors. Top, after a 1-h incubation in the absence or presence of 40 μm VER155008, medium was removed, and cells were washed twice in complete cell culture medium and incubated without inhibitor for the indicated time periods. Bottom, after a 1-h incubation in 10 mm MβCD, cells were washed twice in complete medium and incubated in medium containing 10 μm mevinolin (MβCD/Mevinolin) or in the absence of inhibitors (Mock and MβCD). After an additional 24 h, mevinolin was removed by two medium washes, and cells were incubated for 24 h more (48 h time point). The number of attached extracellular and internalized aggregates was quantified as indicated in A. C, Hsp70 blocking antibodies. cmHsp70.1 antibody was diluted in the culture medium of HEK-293 cells to the indicated concentrations and incubated for 1 h. A solution of PepL was then added to the culture medium to a final concentration of 5 μm. After a 1-h incubation, medium was removed, and cells were washed twice and incubated in complete cell culture medium for the indicated times. The number of attached extracellular and internalized aggregates was quantified as indicated in A. D, membrane Hsp70 staining. HEK-293 cells were either treated with 10 μm geldanamycin or 5 μm peptide PepL-DyLight 550 (red) or left untreated. After an overnight incubation, cells were stained for immunofluorescence with antibody cmHsp70.1-Cy2 (green) and fixed. In samples incubated with aggregates, peptide autofluorescence is responsible for the green signal observed in both antibody-treated and untreated cell samples. Scale bar, 20 μm. E, co-immunoprecipitation of PepL and Hsp70. Biotinylated PepL and purified Hsp70 protein were incubated overnight at 4 °C. The peptide was then precipitated with streptavidin-coupled beads. After SDS-PAGE and Western blot using an anti-Hsp70 antibody, co-precipitation of Hsp70 was detected in the presence of the peptide (right lane). Nonspecific precipitation was not observed in the absence of peptide (left lane), confirming the specific binding between Hsp70 and the peptide.