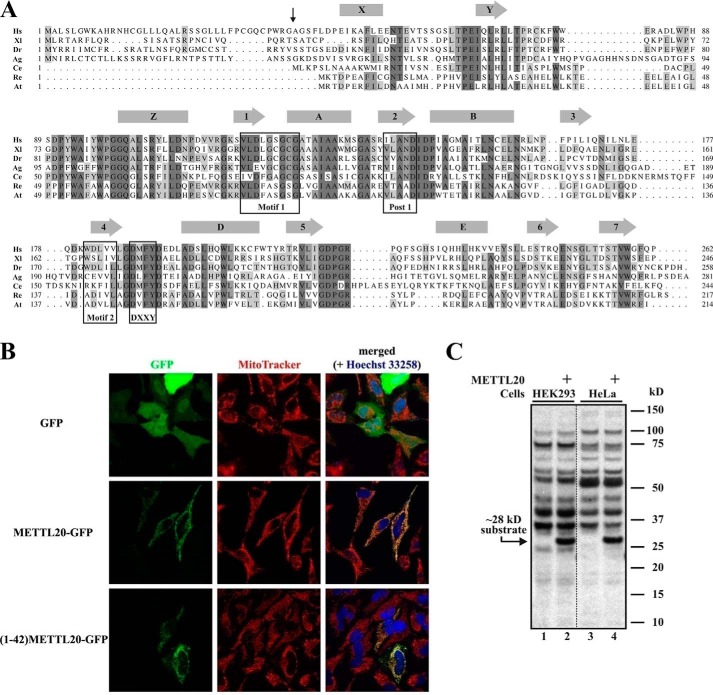
FIGURE 1.
Human METTL20 is an evolutionarily conserved protein methyltransferase localized to mitochondria. A, alignment of METTL20 orthologues from Homo sapiens (Hs; NP_776163.1), Xenopus laevis (Xl; NP_001090037.1), Danio rerio (Dr; NP_001154967.1), Anopheles gambiae (Ag; XP_308218.4), Caenorhabditis elegans (Ce; NP_491943.2), Rhizobium etli (Re; YP_471372.1), and Agrobacterium tumefaciens (At; NP_355584.1). Predicted α-helices (rectangles) and β-strands (arrows) are indicated. Hallmark motifs of 7BS (Motif 1, Post 1, and Motif 2) and MTF16 (DXXY) MTases are boxed. The vertical arrow shows the predicted position of cleavage of putative MTS in human METTL20. B, in vivo confocal fluorescence microscopy images of HeLa cells after 24 h of transient transfection, expressing either GFP, METTL20-GFP, or (1–42)-METTL20-GFP, in the presence of MitoTracker and Hoechst dyes. Data were acquired through green (GFP), red (MitoTracker), and blue (Hoechst) channels and merged. C, recombinant human METTL20 catalyzes methylation of a ∼28-kDa protein in human cell extracts. Extracts from HEK293 or HeLa cells were incubated with [3H]SAM in the absence (lanes 1 and 3) or presence (lanes 2 and 4) of METTL20. Proteins were resolved by SDS-PAGE and transferred onto a PVDF membrane, which was subjected to fluorography. The positions of the ∼28-kDa substrate and molecular mass markers are indicated.