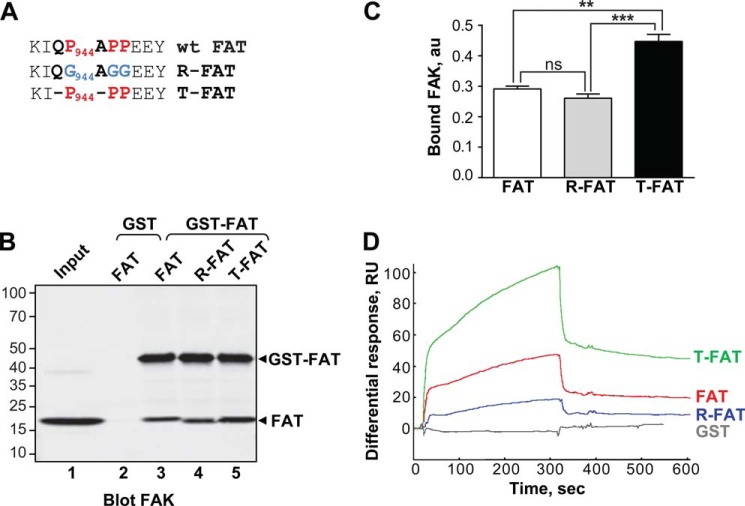
FIGURE 1.
Mutations in the H1-H2 hinge of FAT alter its self-association. A, mutations designed to relax (R) or increase (T) the tension in the HI-H2 FAT hinge region. B, pull-down assays with immobilized GST-FAT or GST and purified FAT domain: WT-FAT (lanes 2 and 3), R-FAT (lane 4), and T-FAT (lane 5). Input (lane 1): 1 μg of soluble FAT equivalent to the total amount used in the pull-down assays. Molecular mass markers positions are indicated in kDa. C, quantification of bound FAT in three independent pull-down experiments (mean ± S.E., a.u., arbitrary units). One-way ANOVA, F2,6 = 37.7, p = 0.0004, Tukey's test **, p < 0.01; ***, p < 0.001; ns, not significant. D, representative surface plasmon resonance sensorgrams showing the binding curves of purified FAT domains to a sensor chip covalently coated with purified GST-FAT. RU, resonance units. Differential response was obtained by subtracting the signal in the blank channel from that in the experimental channel. Estimated Kd: FAT, 23 ± 8.6 μm; T-FAT, 1.3 ± 0.8 μm; R-FAT, 24 ± 5.5 μm (mean ± S.E., n = 3).