FIGURE 4.
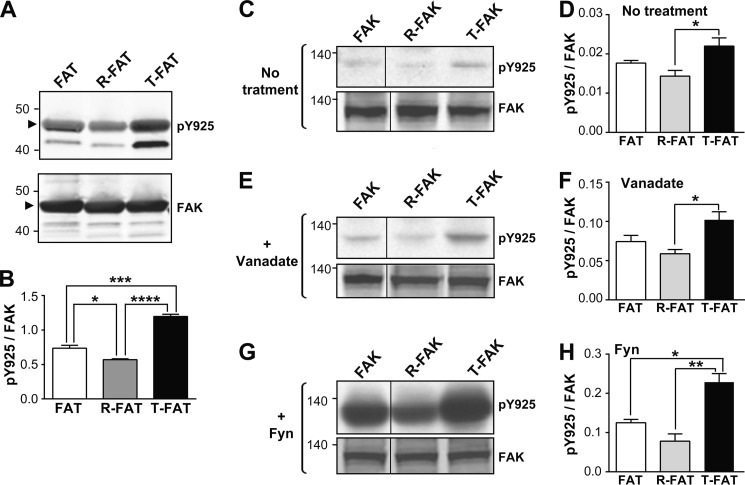
Mutations of the FAT H1-H2 hinge alter phosphorylation of Tyr925. A, purified GST-tagged WT FAT domain (lane 1), R-FAT (lane 2, R-FAT), and T-FAT (lane 3) were incubated with Src (0.1 μg/ml) in the presence of ATP for 15 min at 30 °C. Immunoblotting was carried out with Tyr925 phospho-specific antibody (pY925, upper panel) or a FAK C-terminal antibody (FAK, lower panel). The GST-FAT position is indicated by an arrowhead. Note that phosphorylation of a breakdown product with a lower molecular weight was also affected in the same direction as full-length FAT-GST by the hinge mutations. B, quantification of three experiments for Tyr(P)925 as in A, corrected for the amount of FAK (mean ± S.E.). One-way ANOVA, F(2,6) = 105, p < 0.0001, Tukey's test; *, p < 0.05; ***, p < 0.001; ****, p < 0.0001. C, COS7 cells were transfected with WT or mutated VSV-tagged FAK and pY925 and FAK were analyzed by immunoblotting. D, quantification of results in C. One-way ANOVA, F(2,6) = 6.43, p < 0.05. E and F, same as in C and D, except that cells were treated for 16 h with 50 μm orthovanadate before lysis. One-way ANOVA, F(2,9) = 7.28, p < 0.05. G and H, same as in C and D, except that FAK was cotransfected with B-Fyn. One-way ANOVA, F(2,6) = 19.9, p = 0.002. D, F, and H, Tukey's test, *, p < 0.05; **, p < 0.01. A, C, E, and G, molecular mass markers positions are indicated in kDa. C, E, and G) the samples were run on the same gel and blot but intervening lanes were deleted as indicated by a vertical line.