FIGURE 2.
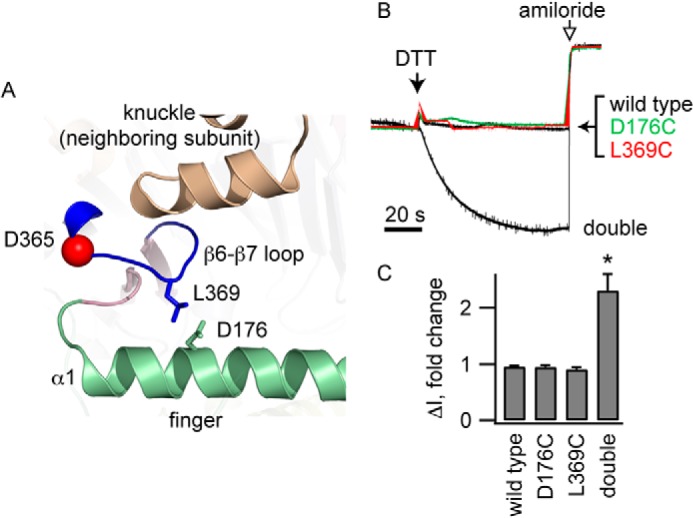
Cross-linking the β6-β7 loop to the α1 helix reduces channel activity. A, Leu-369 on the β6-β7 loop and Asp-176 on helix α1 are adjacent in our model of the α subunit. B, oocytes expressing wild type ENaC, either of the α subunit single mutants (αD176C or αL369C) or the αD176C/L369C double mutant, were exposed to 10 mm DTT and then amiloride (10 μm) while currents were measured at a holding potential of −100 mV. Representative recordings are shown. Currents were normalized to the current immediately prior to DTT addition. Average currents prior to DTT were −1.7 ± 0.4 μA (wild type), −1.5 ± 0.3 μA (αD176C), −1.9 ± 0.4 μA (αL369C), and −1.1 ± 0.2 μA (αD176C/L369C). C, amiloride-sensitive currents following DTT addition normalized to currents prior to DTT (n = 7–10). *, p < 0.01.
