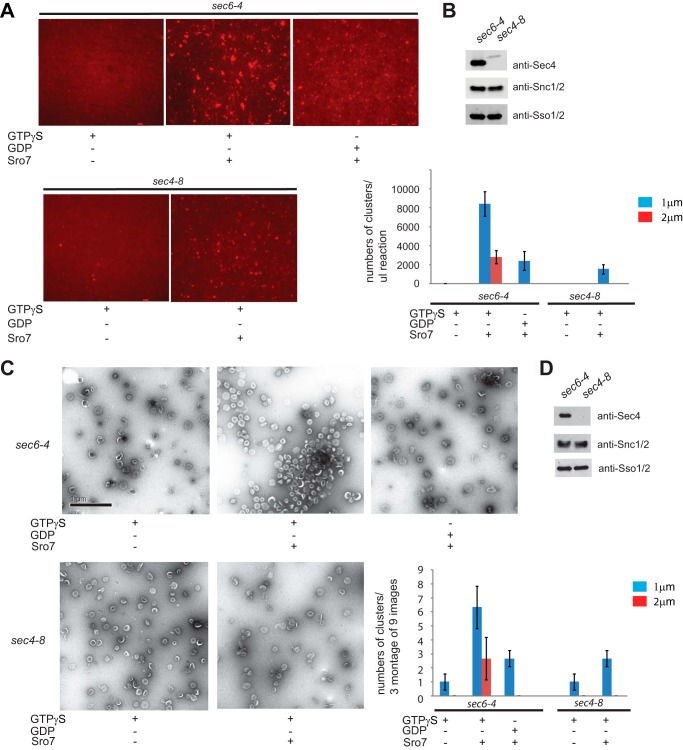
FIGURE 3.
Purified post-Golgi vesicles cluster in vitro in the presence of Sro7, MgCl2, and GTPγS. A, sec6-4 and sec4-8 mutant strains were shifted to the restrictive temperature of 37 °C to accumulate secretory vesicles. Cells were then spheroplasted, lysed, and subjected to a 30,000 × g spin to remove large membranes. The lysates were then treated with FM4-64 and spun through a sorbitol cushion at 100,000 × g to generate two HSP fractions that were then subjected to parallel 20–40% sorbitol velocity gradients. Vesicle-containing fractions were collected from each gradient and subjected to a second 100,000 × g centrifugation to generate a homogeneous fraction of sec6-4 or sec4-8 vesicles. These were then treated with MgCl2 (3 mm) and GTPγS (1 mm) for 30 min on ice and then with Sro7 (1 μm) for 60 min at 27 °C. Clustering was analyzed both by fluorescence microscopy (A) and negative stain electron microscopy (C). HSP fractions of purified vesicles were monitored by Western blot analysis (B and D), and quantitation of vesicle clustering for A was based on counting 10 images at ×60 magnification and separating vesicle clusters by size. Scale bar, 2 μm. Quantitation for C was based on counting three montages of nine frames each.