FIG 7 .
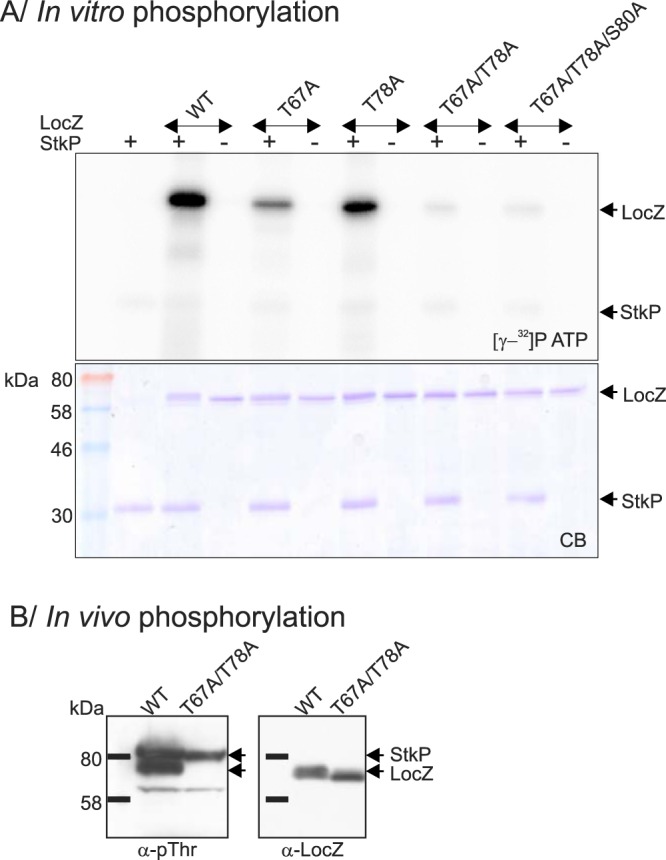
Phosphorylation of LocZ. (A) In vitro phosphorylation. WT and mutated derivatives of the recombinant His6-LocZ protein were subjected to phosphorylation by the recombinant StkP-kinase domain in the presence of [γ-32P]ATP. Samples were resolved by SDS-PAGE, stained with Coomassie blue (CB), and exposed to a sensitive screen ([γ-32P]ATP). The arrows on the right indicate positions of radioactively labeled proteins (upper panel) and Coomassie blue-stained proteins (lower panel). (B) In vivo phosphorylation. Phosphorylation of amino acids T67 and T78 was tested in whole-cell lysates of the WT (Sp208) and the mutant expressing unphosphorylatable LocZ-T67A/T78A (Sp234). Arrows indicate the positions of proteins. Note the difference in the LocZ migration, due to differential phosphorylation.
