Fig. 5.
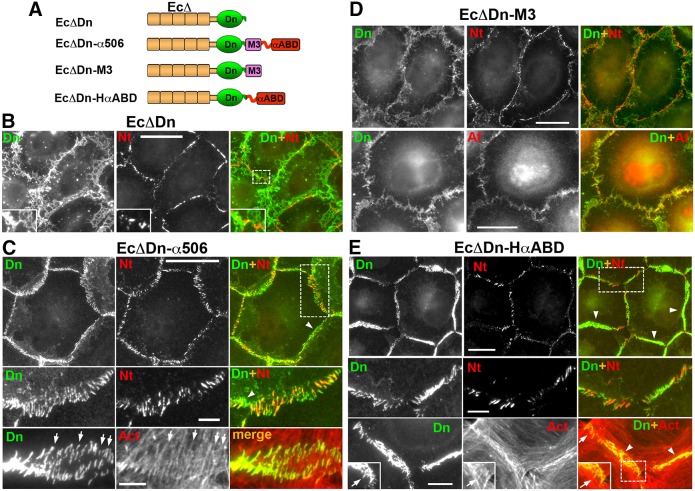
The actin-binding domain of α-catenin is sufficient for nectin–cadherin junction association. (A) Schematic representation of the E-cadherin–α-catenin chimeras. Each chimera includes a different α-catenin region fused with the EcΔDn module consisting of dendra2, Dn (green) and the tailless cadherin, EcΔ (brown), which lacks any known cadherin intracellular binding sites. The C-terminal portion of α-catenin (amino acids 506–906), encompassing the M3 domain and αABD, is present in the EcΔDn–α506 chimera. Two other chimeras, EcΔDn–M3 and EcΔDn–HαABD, incorporate either one of these domains. (B–D) Double immunostaining of A431D cells expressing EcΔDn (B), EcΔDn–α506 (C), EcΔDn–M3 (D), EcΔDn–HαABC (E). All cells were stained with rabbit anti-dendra2 (Dn) to detect the chimeras and mouse anti-nectin-2 (Nt), mouse anti-afadin (Af), or Alexa-Fluor-555–phalloidin (Act). Higher magnifications of the boxed regions (shown in the merged images) are shown either in the insets or in the separate rows. Some of the radial and polymorphic non-radial junctions are indicated by arrows and arrowheads, respectively. Scale bars: 20 µm (5 µm for the zoomed areas).