Fig. 6.
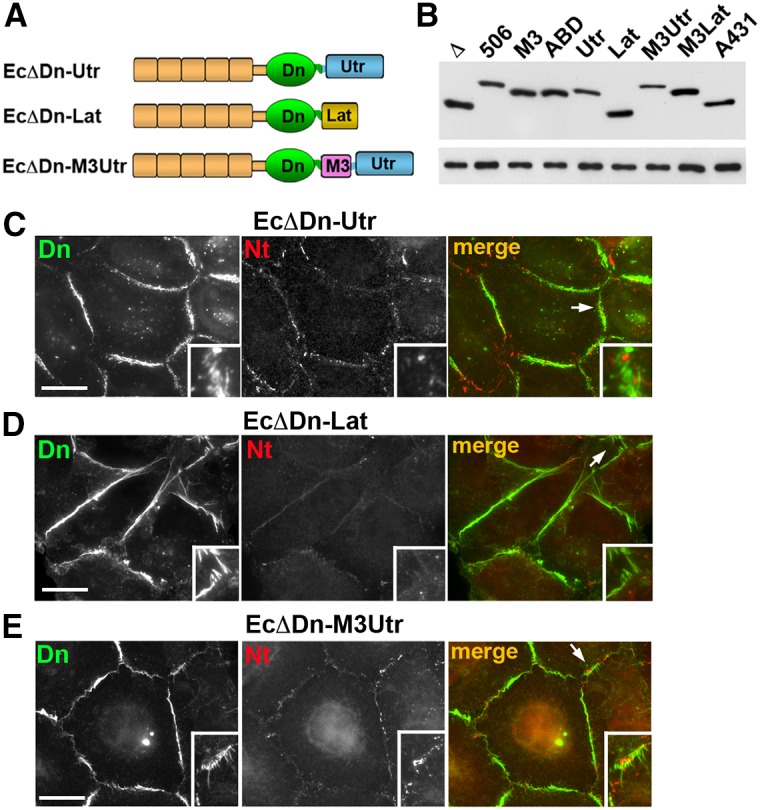
Replacement of the αABD with other actin-binding domains abolishes cadherin–nectin junction association. (A) Schematic representation of non-αABD cadherin chimeras tested. In all cases the cadherin module, EcΔDn, was the same as in the cadherin–α-catenin chimeras (see Fig. 5). The αABD was replaced with the actin-binding domain of utrophin (Utr) in the EcΔDn–Utr chimera or with the actin-binding peptide Lifeact (Lat) in the EcΔDn–Lat chimera. The EcΔDn–M3Utr chimera contained the α-catenin M3 domain in front of the Utr. (B) Immunoblot analysis of the stable cell lines expressing different chimeras: EcΔDn (Δ), EcΔDn-α506 (506), EcΔDn-M3 (M3), EcΔDn-HαABD (ABD), EcΔDn-Utr (Utr), EcDDn-Lat (Lat), EcΔDn-M3Utr (M3Utr), EcΔDn-LatUtr (LatUtr); and the control A431 cells expressing endogenous E-cadherin (A431). Equal amounts of total cell lysates were analyzed using anti-E-cadherin SHE78-7 (upper panel) and anti-tubulin (lower panel) antibodies. (C–E) Double immunostaining of A431D cells expressing EcΔDn-Utr (C), EcΔDn-Lat (D), and EcΔDn-M3Utr (E) using rabbit anti-dendra2 (Dn, green) antibody, to detect the chimeras, and mouse anti-nectin-2 (Nt, red) antibody, to detect nectin junctions. Higher magnifications of the selected regions (indicated by arrows in the merged images) are shown in the insets. Scale bars: 20 µm. Note that the cell–cell contacts enriched with the chimera junctions are mostly devoid of nectin junctions. If present, nectin junctions do not colocalize with the chimeras.
