Fig. 7.
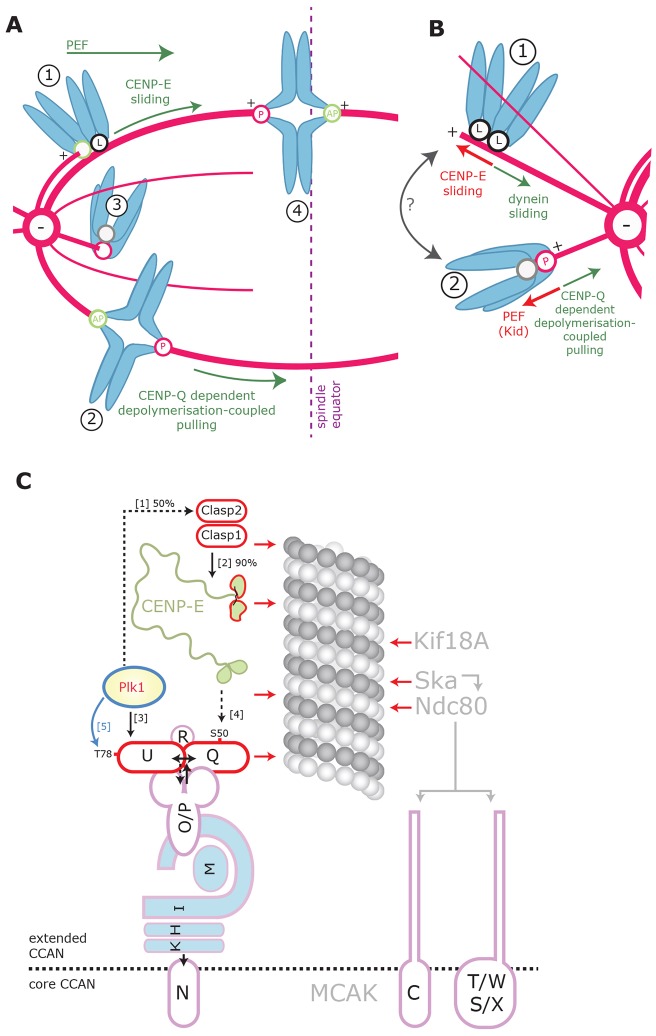
A model for chromosome congression through the combined action of CENP-Q and CENP-E. (A) Schematic showing a proposed model of chromosome congression for unaligned chromosomes positioned between the plate and the pole through the combined action of CENP-Q- and CENP-E-dependent mechanisms. Chromosome 1 is mono-orientated and laterally attached ‘L’ at the black sister kinetochore through CENP-E, this chromosome is able to congress to the plate through lateral sliding, driven by CENP-E, where it is then able to biorientate (as reported in Kapoor et al., 2006). Chromosome 2 is biorientated and able to congress to the metaphase plate by making persistent plateward movements that are driven by CENP-Q-dependent microtubule depolymerisation-coupled pulling at the poleward (P) kinetochore. Chromosome 3 is mono-orientated and will engage its free kinetochore with either the microtubule lattice or the plus-end of microtubules emanating from the opposite pole in order to congress through CENP-E- and/or CENP-Q-dependent pathways. Chromosome 4 is biorientated and aligned at the metaphase plate. AP, away from pole movement. (B) Schematic showing the mechanisms acting on chromosomes positioned behind the spindle poles. Arrows indicate the direction of the generated forces. CENP-E lateral sliding and polar ejection forces (PEFs) move chromosomes anti-poleward, whereas CENP-Q-dependent depolymerisation-coupled pulling and dynein-driven lateral sliding move chromosomes poleward. Force balance amongst these mechanisms dictates the distance of a chromosome from the spindle pole, and kinetochores are probably able to cycle between these attachment states (grey arrow). (C) Schematic showing kinetochore loading and phosphorylation dependencies. Solid black lines represent direct loading dependencies (percentage values indicate the level of dependency). Black dashed lines represent loading dependencies, which are indirect (or have not been shown to be direct). Blue lines represent phosphorylation events, and red arrows represent proteins that have been shown to make direct contact with the microtubule. Percentage dependencies were obtained from the following references: [1] (Maia et al., 2012), [2], (Maffini et al., 2009), [3] (Kang et al., 2006), [4] this study and [5] (Kang et al., 2011). Dependencies within CCAN were obtained from several references (Foltz et al., 2006; McClelland et al., 2007; Okada et al., 2006). The separation of the CCAN into core and extended components is taken from Westhorpe and Straight (Westhorpe and Straight, 2013).