Figure 5.
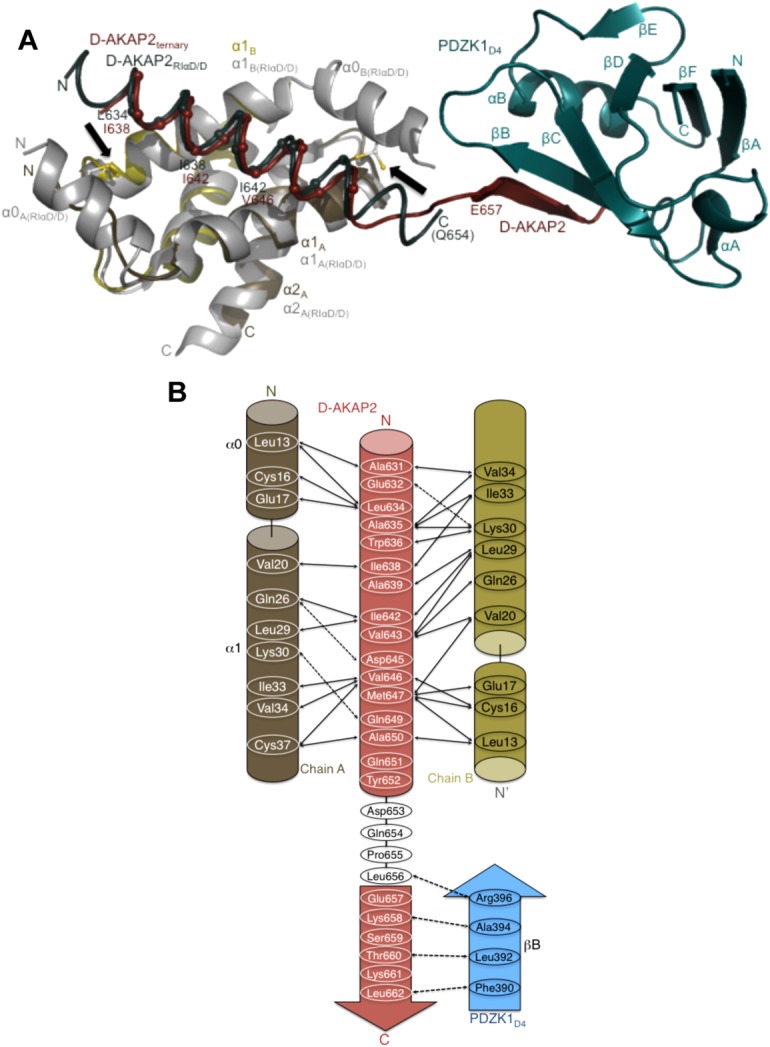
Structural overlay of the ternary complex with the D-AKAP2AKB: RIα D/D and model revealing clues to the reasons for the AKAP helix shift. (a) Structural overlay of the D-AKAP2AKB: RIIα D/D from the ternary complex with the structure of the D-AKAP2AKB: RIα D/D complex crystal structure (PDB ID: 3IM4).13 The ternary complex is colored as Figure 2b. For the D-AKAP2AKB: RIα D/D structure, the D/D domains are colored grey and the D-AKAP2AKB is colored teal. Black arrows indicate the location of the disulfide bonds in the N-terminal helices (α0) of RIα D/D. The helices and termini are labeled with their respective colors. Cα atoms of the D-AKAP2AKB helices are shown and labeled to denote the shift in its helical register when bound to the PKA isoforms. For the ternary complex, the first residue of the β-strand interacting with PDZK1D4, Glu657 is shown. Similarly, the last modeled D-AKAP2 residue in the D-AKAP2AKB: RIα D/D complex, Gln654 is shown and labeled. (b) Schematic figure detailing the potential binding mechanism of PDZK1D4 and RIα D/D to the D-AKAP2AKB. Only with the helical shift would D-AKAP2 be able to bind to PKA and PDZK1 simultaneously. Solid and dashed arrows indicate the hydrophobic and polar interactions respectively in the D-AKAP2AKB: RIα D/D crystal structure and the potential interactions between D-AKAP2AKB and PDZK1D4.13.
