Figure 5.
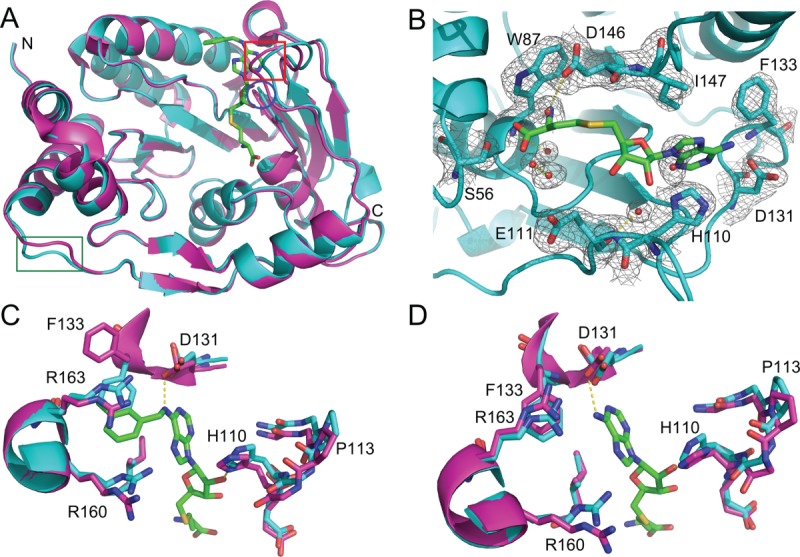
Refolded and native DNV3 MTase have similar crystal structures. A: Alignment of the A-chains of native (magenta, PDB 3P8Z) and refolded (cyan) DNV3 MTase crystal structures. Loops where the structures differ (residues 80–84, 104–111, and 243–247) are indicated by a blue circle, red box, and green box, respectively. B: The 2Fo-Fc electron density map of the refolded DNV3 MTase SAM-binding site at 2.1 Å resolution, contoured at 1.08 σ level (0.2701 e/Å3) above the mean density. SAH from the native structure B-chain is added to the SAM-binding site for reference and is colored by element, carbon, green; nitrogen, blue; oxygen, red; sulfur, yellow. C: Active sites from the aligned native (magenta) and refolded (cyan) DNV3 MTase A-chains. The SAH-derived compound present in the 3P8Z A-chain is colored as SAH in (B). D: As in (C), but SAM-binding sites are from the aligned B-chains. SAH is present in the 3P8Z B-chain and is colored by element as in (B).
