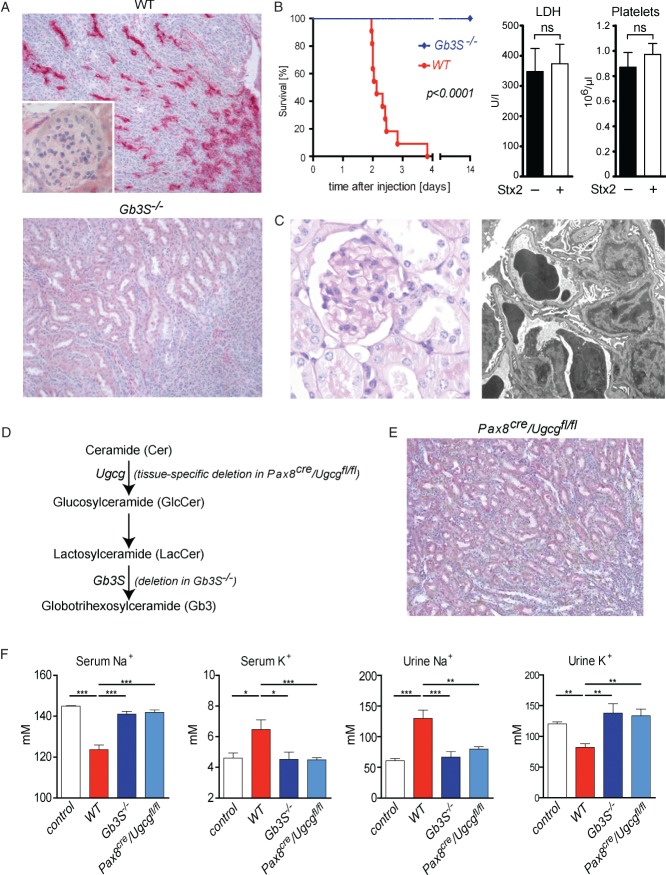
Figure 1.
Stx2 exerts direct toxic effects towards tubular epithelial cells. (A) Indirect immunohistochemistry using primary anti-Gb3 antibodies in combination with alkaline phosphatase-conjugated secondary antibodies visualized Gb3 expression in collecting ducts but not in glomerular or renal endothelial cells of WT mice. In Gb3S-deficient (Gb3S−/−) mice, Gb3 expression was completely abolished; magnification = ×200. (B) WT and Gb3S−/− mice were injected with 0.2 µg Stx2 i.p. While all WT mice died 2–4 days after the injection, all Gb3S−/− mice survived and showed no abnormalities. In moribund Stx2-injected WT mice, serum lactate dehydrogenase (LDH) and platelet counts were measured 36 h after the injection of Stx2. No increase in LDH or decrease of platelet count could be observed, as compared to PBS-injected control WT mice (n = 5/group). (C) Consistent with the absence of Gb3 in the murine glomerulus, TMA could not be detected in the glomeruli of moribund mice. Upon light and transmission electron microscopy, the glomerular morphology was unremarkable; in particular, no signs of endothelial damage or TMA could be detected by electron microscopy: (left panel) PAS staining, magnification = ×400; (right panel) electron micrograph, lead citrate/uranyl acetate staining, magnification = ×6300. (D) Scheme of the synthesis of globotrihexosylceramide (Gb3), starting with ceramide (Cer). Successive additions of one glucose (Glc) and two galactose moieties result in the formation of Gb3. The last step is catalysed by Gb3 synthase (Gb3S) and is defective in Gb3S−/− mice. Tissue-specific depletion of Gb3 was achieved by deletion of the UDP-glucose:ceramide glucosyltransferase (Ugcg) gene, which encodes for the enzyme catalysing the synthesis of glucosylceramide (GlcCer) two steps upstream of Gb3S. (E) Immunohistochemistry for Gb3 performed as in (A), revealed no tubular staining in the kidneys of Pax8cre/Ugcgfl/fl mice as contrasted to WT (cf A); magnification = ×200. (F) 36 h after the Stx2-injection, serum and urine analysis showed profound and significant hyponatraemia and hyperkalaemia mirrored by inverse changes in urine sodium and potassium in WT mice. In contrast, global (Gb3S−/−) as well as tubule-specific depletion of Gb3 (Pax8cre/Ugcgfl/fl) were fully protective. PBS-injected mice served as controls (n = 5/group); bars in (B, F) show mean ± SEM; statistical differences were tested by two-tailed Student's t-test; ns, non-significant; *p < 0.05; **p < 0.01; ***p < 0.001