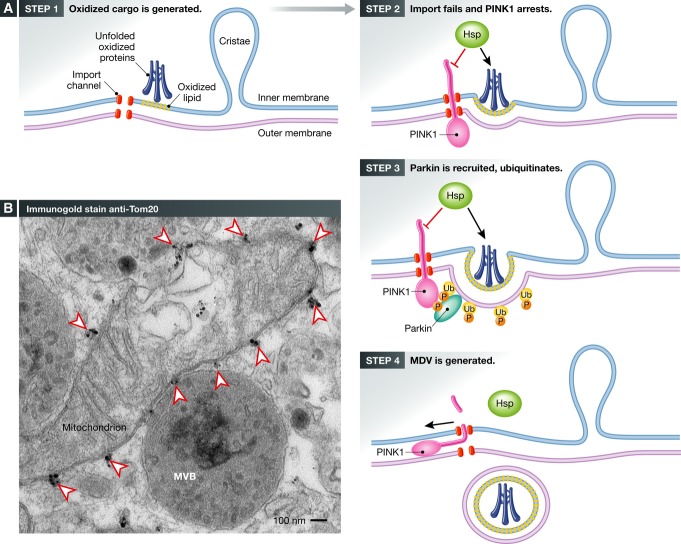
Figure 2. Working hypothesis for vesicle initiation by PINK1 and Parkin.
(A) Immunogold staining of endogenous Tom20 within COS7 cells reveals the regular spacing of the import channels indicated by arrowheads. Note the close tethering of three multivesicular bodies to the mitochondria. (B) An illustration of our working hypothesis of PINK1/Parkin-mediated MDV formation. In Step 1, unfolded, oxidized proteins within matrix, triggered by ROS or failure to assemble, leads to protein aggregation (blue). Oxidation of cardiolipin will generate PA, contributing to altered membrane curvature. In Step 2, protein aggregates may saturate chaperones, leading to a very localized failure to import at an individual channel. In addition, local oxidation of cardiolipin would further interfere with import channels. PINK1, which is rapidly imported, would then accumulate at these failed import channels. In Step 3, PINK1 phosphorylates both ubiquitin and the ubiquitin-like domain of Parkin, stabilizing the recruitment of activated Parkin. The ubiquitination activity of Parkin is required to generate MDVs, suggesting that domains on the surface may be cleared. In Step 4, a vesicle is formed and released in a process that will certainly involve a number of unidentified proteins. Future studies are needed to test this hypothesis and uncover the details governing the generation of MDVs.