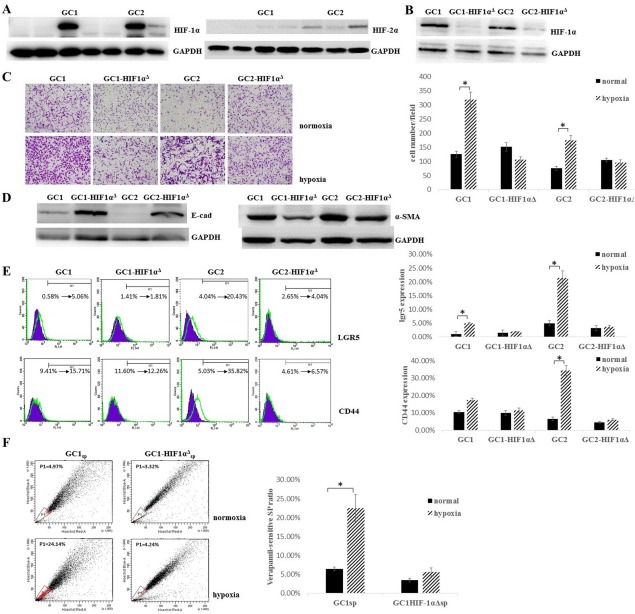
Figure 2.
Hypoxia induced epithelial-mesenchymal transition in gastric cancer cells (GCCs) and enhanced the gastric cancer stem/progenitor cell ratio in GCCs through HIF-1α. (A): GCCs were primary cultured from seven patients who exhibited gastric cancer peritoneal dissemination, HIF-1α and HIF-2α expression were analyzed by Western blot after 48 hours hypoxic culture. GAPDH served as a protein loading control. (B): GC1 and GC2 were stably transfected with HIF-1α shRNA (GC1HIF-1αΔ and GC2HIF-1αΔ) or scramble sequence (GC1 and GC2), HIF-1α expression was analyzed by Western blot after 48 hours hypoxic culture. GAPDH served as a protein loading control. (C): GC1, GC2, GC1HIF-1αΔ, and GC2HIF-1αΔ were cultured under normoxic or hypoxic condition, migration ability was analyzed by Boyden chamber migration assay, cells were fixed with 4% paraformaldehyde and stained with 1% crystal violet (magnification ×200, cell number per field was expressed as mean ± SD, n = 4, *, p < .05). (D): Epithelial-mesenchymal transition-related protein E-cadherin and α-smooth muscle actin expression were analyzed by Western blot. GAPDH served as a protein loading control. (E): GC1, GC2, GC1HIF-1αΔ, and GC2HIF-1αΔ were cultured under normoxic or hypoxic condition, surface staining of cancer stem cells related proteins CD44 and lgr5 was analyzed by fluorescence-assisted cell sorting (FACS, ratio of positive stained cell were expressed as mean ± SD, n = 4, *, p < .05, purple plots indicated GCCs cultured under normoxia and green plots indicated GCCs cultured under hypoxia). (F): Verapamil-sensitive SP ratio in GC1 and GC1HIF-1αΔ was analyzed by FACS, cells were cultured under hypoxic or normoxic conditions for at least 7 days (ratio of SPs was expressed as mean ± SD, n = 3, *, p < .05). Abbreviation: HIF-1α, hypoxia-inducible factor-1α.