Figure 1. Structure of the tmRad50NBD–Mre11HLH–DNA complex.
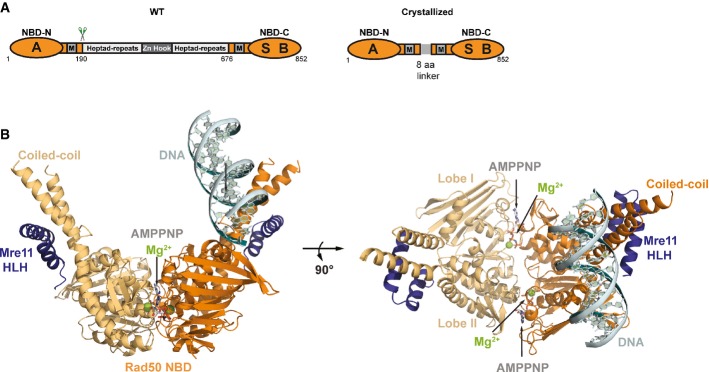
- Domain structure of wild-type Rad50 (WT, left) and the crystallized Rad50NBD construct (right). RAD50 contains a bipartite ATP-binding cassette-type nucleotide-binding domain (NBD, orange) consisting of N-terminal (NBD-N) and C-terminal (NBD-C) segments. The N-terminal segment harbors the Walker A motif (A); the C-terminal segment harbors the Walker B (B) and signature motifs (S). M: Mre11 binding sites. NBD-N and NBD-C are at the ends of a heptad-repeat segment that forms an antiparallel coiled-coil. The center of the heptad-repeat segment contains the Zn-hook dimerization motif.
- Ribbon representation with highlighted secondary structure of the nucleotide-binding domain (NBD) dimer of Rad50 (yellow and orange) in complex with the Mre11 C-terminal helix-loop-helix (HLH) motif, Mg2+-AMPPNP (Mg2+: green sphere, AMPNP: gray-color-coded sticks), and double-strand DNA (cyan ribbon and sticks) shown in two orientations. Rad50 dimerizes in the typical head-to-tail arrangement, sandwiching two Mg2+-AMPPNP moieties in the dimer interface. The DNA binds to a strand-loop-helix motif on one NBD of Rad50 and additional contacts are observed to the adjacent coiled-coil.
