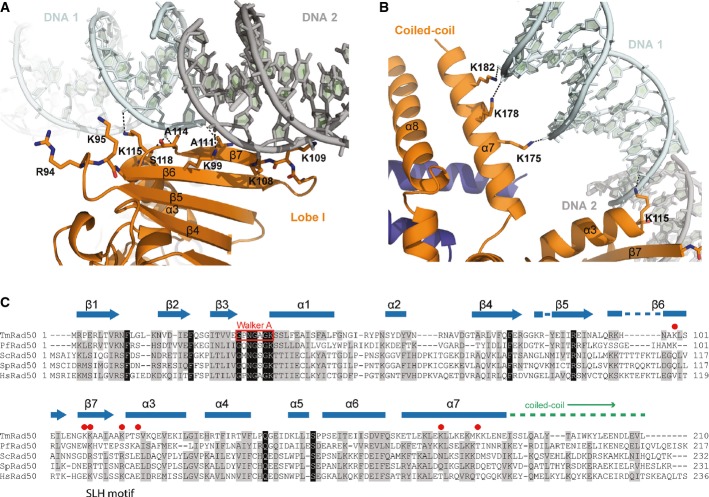
Detailed view of the DNA interactions with the Rad50 NBD (orange) shown in ribbon representation with the contacting residues highlighted as sticks. DNA contacts involve interactions of the strand-loop-helix motif of the Rad50 dimer (residues K99, K108, K109, A111, A114, K115, and S118) with the sugar–phosphate backbone of the two DNA molecules (cyan and gray). The second DNA molecule, depicted in gray, belongs to the symmetry-related molecule and forms a quasi-continuous DNA strand in the crystal structure. Residues R94 and K95 are in close proximity of the DNA-binding region and evidently involved in DNA binding.