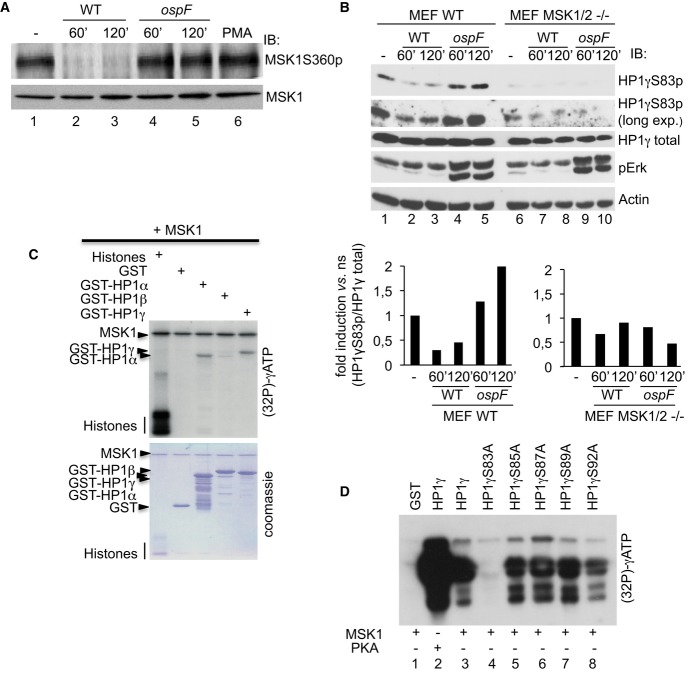
Figure 4. The MSK1 kinase drives HP1γS83p formation in Shigella-infected cells.
A OspF leads to the accumulation of a dephosphorylated and inactive form of MSK1 into Shigella-infected cells. HeLa cells were infected with the Shigella flexneri invasive (WT) or the ospF-deficient (ospF) strains at the indicated times or PMA (60 min). Western blots were performed with anti-MSK1S360p or MSK1 antibodies.
B The MSK1 kinase modulates the HP1γS83p signal in Shigella-infected cells. Primary MEFs WT and MSK1/2 double null (MEF MSK1/2−/−) were infected with the Shigella flexneri invasive strain (WT) or the OspF-deficient strain (ospF) at the indicated times. Western blots were performed with anti-HP1γS83p, anti-HP1γ, anti-phospho-ERK, and anti-actin antibodies. Lower panel: Densitometric quantification of the signal obtained by Western blot. For each time point, the results are expressed as the level of HP1γS83p signal related to the total amount of HP1γ.
C, D Kinase assays showing that MSK1 directly phosphorylates HP1γ at the S83 residue. Purified active MSK1 was incubated with histones, GST or GST-HP1α, GST-HP1β, GST-HP1γ or the indicated GST-HP1γ mutant fusion proteins. Kinase assay was performed at 30°C during 1 h in the presence of [γ-32P]-ATP followed by autoradiography.
Source data are available online for this figure.