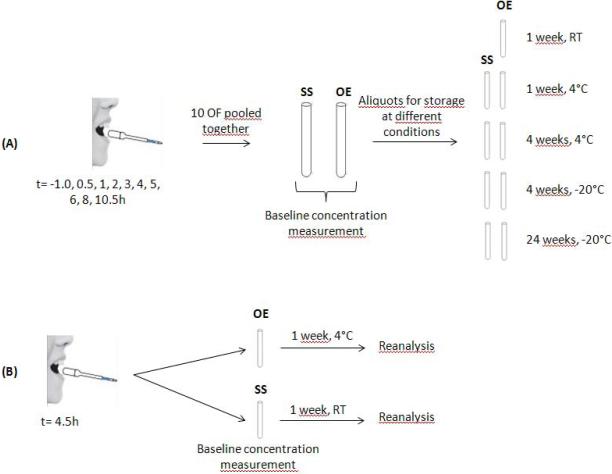
Figure 1.
Stability evaluation design. (A) For each participant, one collection with each device at each time point (t= -1.0, 0.5, 1, 2, 3, 4, 5, 6, 8, 10.5h ) was performed resulting in 10 different OF for each device. After individual analyses for pharmacokinetics purposes, the remaining OF from each of 10 OF were pooled in one tube for each device (StatSure=SS and Oral-Eze=OE) which was analyzed within 24h for baseline concentrations. After baseline quantification, different aliquots were stored under different conditions for different durations for stability analyses. (B) To evaluate manufacturers’ recommended storage conditions, the 4.5h time point was collected with each device and analyzed within 24h for baseline concentration. After analysis, the tubes were kept for 1 week at 4° (Oral-Eze) or room temperature (StatSure), analyzed and compared to baseline for stability purpose.