Abstract
Background/Aims
We investigated the clinical outcomes according to the method of treatment in synchronous esophageal and gastric cancer.
Methods
Synchronous esophageal squamous cell carcinoma and gastric adenocarcinoma were diagnosed in 79 patients between 1996 and 2010. We divided the patients into four groups according to treatment; Group 1 received surgical resection for both cancers or surgery for gastric cancer with chemoradiotherapy for esophageal cancer (n=27); Group 2 was treated by endoscopic resection with or without additional treatment (n=14); Group 3 received chemoradiotherapy only (n=18); and Group 4 received supportive care only (n=20).
Results
The median survival times in groups 1 and 2 were 86 and 60 months, respectively. The recurrence rate and mortality were 23% and 48%, respectively, in group 1 and 21% and 4%, respectively, in group 2. The median survival time was 12 months in group 3 and 9 months in group 4. Multivariate analysis showed that age (p<0.001) and treatment group (p=0.019) were significantly associated with death. Compared with group 1, treatment in the intensive care unit (p=0.003), loss of body weight (p=0.042), and decrease in hemoglobin (p=0.033) were worse in group 1.
Conclusions
Endoscopic resection for synchronous esophageal and gastric cancer could be considered as a possible alternative to surgery for early-stage cancer.
Keywords: Synchronous, Esophageal neoplasms, Stomach neoplasms
INTRODUCTION
The occurrence of multiple primary cancers in the upper aero-digestive tract including the esophagus is a well-known phenomenon that has been explained by the concept of “field cancerization.” However, the occurrence of synchronous esophageal squamous cell carcinoma and gastric adenocarcinoma is rare (1.4% to 5.8%).1,2 The reason for its rarity is thought to be that the two cancers do not share risk factors; for example, smoking has been identified as a definite risk factor for esophageal squamous cell carcinoma, while Helicobacter pylori infection is an important risk factor for gastric adenocarcinoma. Recently, an increase in the number of diagnosed multiple primary cancers has been reported,3 and the frequency of synchronous upper gastrointestinal tract cancer is also increasing due to the application of advanced diagnostic tools, especially endoscopy, and the prolonged life span of the general population.
There have been a few reports of synchronous esophageal and gastric carcinoma. In 1998, Koide et al.2 reported retrospectively on 24 cases of synchronous gastric tumors associated with esophageal cancer, but not all the gastric tumors were malignant. More recently, Bai et al.4 described 64 synchronous upper gastrointestinal malignancies including 34 cases of synchronous esophageal squamous cell carcinoma and gastric adenocarcinoma. However, there was insufficient discussion of treatment and prognosis in that study, and the number of patients was too small for an analysis of the clinicopathological and endoscopic features. To our knowledge, there have been few studies of the prognosis of these synchronous cancers according to treatment modality including endoscopic resection. Moreover, there has been only one report, involving two cases, of endoscopic treatment for synchronous esophageal and gastric cancer.5
We, therefore, aimed to investigate the clinical outcomes of synchronous esophageal and gastric cancers in a single center according to treatment method.
MATERIALS AND METHODS
1. Patients
Between December 1996 and December 2010, a total of 2,405 patients with histologically confirmed esophageal carcinoma, and 26,029 patients with histologically confirmed gastric carcinoma were admitted to Asan Medical Center, and synchronous esophageal and gastric carcinomas were found in 93 patients. Of these, 14 patients were excluded, including two patients with esophageal adenocarcinoma, which is relatively rare in Korea, six with gastric squamous cell carcinoma, and six with incomplete data. Therefore, we analyzed the outcomes of 79 patients with synchronous esophageal squamous cell carcinoma and gastric adenocarcinoma. After getting verbal informed consent, we obtained information about the status of each patient, including the cause of death of any patient who was lost to follow-up. This study was approved by the Institutional Review Board of Asan Medical Center.
2. Definitions
A gastric cancer was defined as synchronous if it occurred within 6 months of diagnosis of the esophageal cancer. Curative treatment was defined as no tumor after treatment of an esophageal cancer in follow-up examination with endoscopy, chest computed tomography, and/or positron emission tomography scan, which was performed after March 2001 in our hospital. Tumor staging was based on the American Joint Committee on Cancer 7th TNM system and the degree of differentiation was classified as recommended by the World Health Organization.6 Well or moderately differentiated carcinomas were classified as differentiated histologic type. Poorly differentiated carcinoma and signet ring cell carcinomas were classified as undifferentiated types. If the two types were mixed, we selected the dominant one, as recommended by the World Health Organization.7
3. Treatment protocols for resectable esophageal cancer and gastric cancer in our hospital
Before 1997, neoadjuvant chemoradiotherapy (CRT) was performed for esophageal cancer and the tumor was then reevaluated for resectability. Between 1999 and 2002, immediate surgery or neoadjuvant CRT was performed for resectable cancer. After 2003, induction chemotherapy followed by CRT was performed in resectable esophageal cancer. Surgical resection was performed 4 to 6 weeks after the end of radiotherapy (RT) using a transhiatal abdominal-right thoracic (Ivor-Lewis) or right thoracic-abdominal-cervical (McKeown) approach. The proximal and distal margins had to be ≥6–8 cm from the gross tumor. A frozen section of the resection margin of each tumor was examined by a pathologist before completion of surgery. Resection was considered incomplete when microscopic examination revealed a positive margin (R1) or residual gross disease (R2). Patients who had incomplete surgical resection were treated with additional postoperative RT, with or without chemotherapy.8
Gastrectomy was recommended for patients who do not fulfill the criteria for endoscopic resection. Gastrectomy removing both the tumor and the lymph nodes was also being performed where lymph node involvement was detected or strongly suspected during preoperative staging. Either subtotal or total gastrectomy was performed, depending upon the tumor location, and laparoscopic-assisted gastrectomy has been performed in our institute since 2004.
Endoscopic resection was performed in some cases of superficial esophageal cancer including mucosal esophageal cancer (T1m) without evidence of lymph node metastasis, and in differentiated early gastric cancer without evidence of lymph node metastasis within the indication criteria, including (1) mucosal cancers without ulcerative findings, regardless of tumor size; (2) mucosal cancers with ulcerative findings ≤30 mm; and (3) a minute (<500 μm from the muscularis mucosae) submucosal invasive cancers ≤30 mm.9,10 Only endoscopic mucosal resection (EMR) was performed until 2003, and, in addition, endoscopic submucosal dissection (ESD) was performed after 2004. In EMR, after checking the lesion, saline containing epinephrine mixed with indigo carmine was injected into the submucosal layer. The raised lesion was removed using an SD-9U-1 or SD-12U-1 snare (Olympus, Tokyo, Japan) after circumferential mucosal incision. For ESD, the typical procedure sequence involved marking, mucosal incision, and submucosal dissection with simultaneous hemostasis. After making several marking dots outside the lesion, saline containing epinephrine and indigo carmine was injected into the submucosal layer. A circumferential incision was made and submucosal dissection was performed with various knives until the lesion was completely removed. Endoscopic hemostasis was performed with hemoclips or hemostatic forceps (FD-410LR; Olympus) whenever bleeding or an exposed vessel was observed.
4. Subgroup analysis
In order to compare the clinical outcomes of the various treatment options for synchronous cancer, the patients were divided into four groups according to treatment method. Patients who were treated with curative intent were divided into group 1 and group 2 to compare endoscopic and surgical resection. Group 1 received surgical resection for both cancers or surgery for gastric cancer with CRT for esophageal cancer (n=27), group 2 were treated with endoscopic resection for at least one cancer with or without some other treatment, such as surgery and CRT (n=14), group 3 received only CRT without surgical or endoscopic resection (n=18), and group 4 were given only supportive care (n=20).
5. Statistical analysis
Baseline continuous and categorical variables are presented as median and number (percentage). Continuous variables were compared using Student t-test or the Wilcoxon rank-sum test, and categorical variables were compared with Fisher exact test or Pearson chi-square test. Correlations between variable factors and death were assessed by univariate and multivariate Cox proportional hazards regression analysis. Variables with a p-value <0.15 in univariate analysis were included in the multivariable model. The final models were determined by backward variable selection, where the least significant variables were removed one-by-one after fitting the full nonparsimonious model. All p-values were two-sided, and p-values less than 0.05 were considered statistically significant. R software version 2.10.1 was used for all statistical analyses.
RESULTS
1. Baseline characteristics of synchronous esophageal squamous carcinoma and gastric adenocarcinoma
Baseline characteristics of the 79 patients are presented in Table 1. The median survival time was 21 months (interquartile range [IQR], 8 to 81 months) for all 79 patients (77 men and two women: median age, 67 years; IQR, 61 to 71 years). Of these, 34 had underlying disease, with 21 presenting with cardiovascular disease (16 with hypertension, three with ischemic heart disease, and two with atrial fibrillation). In addition, six patients presented with diabetes mellitus and two with neurological disease (both of whom had suffered previous cerebral vascular accidents). Furthermore, seven had a history of another form of cancer (three with head and neck cancer, two with uterine cancer, and one each with prostate and skin cancer). We also found that three patients had liver cirrhosis, two had tuberculosis-associated lung disease, and one had a history of pancreatitis. Sixteen patients had a family history of malignancy (eight with gastric cancer, five with lung cancer, two with liver cancer, and one with colorectal cancer).
Table 1.
Demographic and Clinical Characteristics of the 79 Patients with Synchronous Esophageal and Gastric Cancer
| Characteristic | Gastric cancer | Esophageal cancer |
|---|---|---|
| Age, yr | 67 (61–71) | |
| Male sex | 77 (97.5) | |
| History of smoking | 66 (83.5) | |
| History of alcohol consumption | 67 (84.8) | |
| Family history of cancer | 16 (20.3) | |
| Tumor location | ||
| Upper | 22 (27.8) | 10 (12.7) |
| Middle | 27 (34.2) | 14 (17.7) |
| Lower | 30 (38.0) | 55 (69.6) |
| Histology of cancer | ||
| Differentiated* | 53 (67.1) | 57 (72.2) |
| Undifferentiated† | 26 (32.9) | 22 (27.8) |
| Stage of cancer (TNM)‡ | ||
| I | 49 (62.0) | 20 (25.3) |
| II | 14 (17.7) | 26 (32.9) |
| III | 15 (19.0) | 21 (26.6) |
| IV | 1 (1.3) | 12 (15.2) |
| Treatment | ||
| Surgery | 27 (34.2) | 25 (31.6) |
| Endoscopic resection | 13 (16.5) | 4 (5.1) |
| Chemoradiotherapy | 0 | 28 (35.4) |
| Conservative | 39 (49.4) | 22 (27.8) |
Data are presented as number of patients (%) or median (interquartile range).
Differentiated: well or moderately differentiated;
Undifferentiated: poorly differentiated, undifferentiated, or signet ring cell;
Based on the American Joint Committee on Cancer 7th edition TNM system.
Of the 79 patients, 59 were diagnosed as synchronous esophageal and gastric cancer at first endoscopy. Of the remaining 20 patients, four patients could not be diagnosed with gastric cancer initially because the endoscope was not able to pass through a stricture, in eight others the early gastric cancer was missed at the initial EGD, and in the remaining eight superficial esophageal cancers were missed initially. At the initial EGD, a total of six patients had strictures that made it impossible for the endoscope to pass the esophagus. In two of these patients the gastric cancer was diagnosed using an ultra-slim scope, in two others after insertion of an esophageal stent, in the fifth after resolution of the stricture by CRT, and in the final patient by examination of a surgical specimen.
2. Treatment of synchronous cancers and clinical outcomes
The incidence of synchronous esophageal squamous cell carcinoma and gastric adenocarcinoma has increased with time and the treatment modality has changed. In addition, the number of individuals recommended for screening for gastric cancer in Korea increased from 403,000 to 3,021,000 between 2002 and 2011.11
The treatments employed for synchronous esophageal and gastric cancer are shown in Table 2. Of the 29 cases where both cancers were resected, surgical resection was performed for both cancers in 20 cases, one cancer was treated by surgical resection and the other by endoscopic resection in six cases, and both cancers were treated by endoscopic resection in three cases. Surgical or endoscopic resection for the gastric cancer combined with CRT for the esophageal cancer was performed with curative intent in 12 patients.
Table 2.
Treatments for Synchronous Esophageal and Gastric Cancer
| Gastric cancer | Esophageal cancer | |||
|---|---|---|---|---|
|
| ||||
| Surgery (±CRT) | ESD | CRT | None | |
| Surgery | 20 | 1 | 7 | |
| Mortality | 10 (50.0) | 0 | 3 (43.0) | |
| Follow-up period, mo | 37.4 (18.3–75.9) | 59 | 52.9 (26.5–71.8) | |
| Survival period, mo | 87.7 (23.8–104.0) | - | 54.1 (52.9–103.7) | |
| ESD | 5 | 3 | 5 | |
| Mortality | 0 | 0 | 4 (80.0) | |
| Follow-up period, mo | 29.1 (25.8–52.8) | 19.3 (14.7–22.0) | 19.7 (12–24.8) | |
| Survival period, mo | - | - | 14.2 (12–42.8) | |
| None | 18 | 20 | ||
| Mortality | 17 (94.0) | 20 (100.0) | ||
| Follow-up period, mo | 11.7 (5.9–19.8) | 9.7 (3.9–16.7) | ||
| Survival period, mo | 8.1 (5.9–19.7) | 8.0 (3.0–15.0) | ||
Data are presented as number of patients (%) or median (interquartile range).
CRT, chemoradiotherapy; ESD, endoscopic submucosal dissection.
Negative resection margins were confirmed in the surgical specimens of all 25 patients who received surgical resection of the esophageal cancer, but recurrence was detected in five of these patients a median 22.4 months (IQR, 11.5 to 55.4 months) after treatment; one of these five patients developed metastatic hepatic and bony lesions 6 months after operation, and four received chemotherapy for their recurrence. Four of the five patients died a median of 33 months (IQR, 14.5 to 102.5 months) from the initial diagnosis, and one patient has been followed up for 8 years after chemotherapy without further recurrence.
Complete resection was achieved in all four patients who received endoscopic resection of their esophageal cancer. Two of these suffered recurrence 8 and 20 months after treatment, respectively; both received endoscopic resection once more, and have been followed for 13 and 32 months, respectively, without further recurrence.
Complete resection was also achieved in 12 of the 13 patients who received endoscopic resection of their gastric cancer, and the remaining patient with involvement of the lateral or vertical margin was treated with chemotherapy. In three of these patients, other early gastric cancers were found, 4, 6, and 12 months, respectively, after the first EMR of their gastric cancer. Of these three patients, one was treated by endoscopic resection, one underwent a total gastrectomy, and one refused further treatment.
The overall 3- and 5-year survival rates of all patients were 36% and 29%, respectively in group 1; the overall 3- and 5-year survival rates were 69% and 52%, respectively in group 2; they were both 57% in group 3; the 3-year survival rate was 7%, and all patients in group 4 died within 3 years. The various oncologic outcomes are presented in Table 3.
Table 3.
Oncological Outcomes of Patients according to Treatment Group
| Total (n=79) | Group 1 (n=27) | Group 2 (n=14) | Group 3 (n=18) | Group 4 (n=20) | |
|---|---|---|---|---|---|
| Cure after initial treatment | 37 (46.8) | 22 (84.5) | 10 (71.4) | 5 (27.8) | 0 |
| Recurrence | 10 (12.7) | 6 (23.1) | 3 (21.4) | 1 (5.6) | - |
| Recurrence time, mo | 24 (7–35) | 24 (16–34) | 21 (14–29) | 28 | - |
| Deaths | 53 (67.1) | 13 (48.1) | 4 (28.6) | 17 (94.4) | 20 (100.0) |
| Survival time, mo | 21 (8–81) | 86 (39–103) | 60 (43−) | 12 (5–19) | 9 (3–15) |
Data are presented as number of patients (%) or median in months (interquartile range).
3. Risk factors for death from synchronous esophageal and gastric cancer
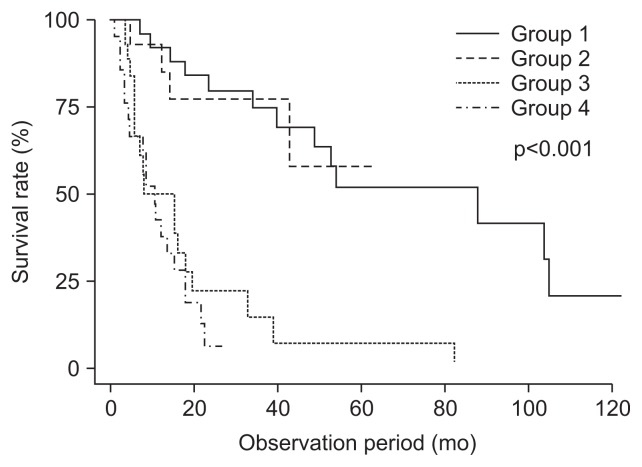
Univariate analysis showed that older age, advanced histology of esophageal cancer, advanced stage of esophageal and gastric cancer, and membership of group 3 or 4 increased death (Table 4). In multivariate analysis, age (hazard ratio [HR], 1.076; 95% confidence interval [CI], 1.033 to 1.120; p<0.001) and membership of group 3 or 4 (HR, 4.058; 95% CI, 1.559 to 10.564; p=0.004 and HR, 3.310; 95% CI, 1.124 to 9.751; p=0.030) increased death, while no significant difference in risk of death was found between group 1 (reference) and group 2 (HR, 1.153; 95% CI, 0.335 to 3.965; p=0.821) (Table 5, Fig. 1).
Table 4.
Univariate Analysis of the Risk Factors for Death
| Hazard ratio | 95% CI | p-value | |
|---|---|---|---|
| Age | 1.061 | 1.020–1.103 | 0.004 |
| Location of esophageal cancer | |||
| Upper | Reference | ||
| Middle | 8.667 | 0.790–95.09 | 0.077 |
| Lower | 1.079 | 0.272–4.278 | 0.913 |
| Histology of esophageal cancer | |||
| Differentiated* | Reference | ||
| Undifferentiated† | 2.574 | 1.432–4.628 | 0.002 |
| Histology of gastric cancer | |||
| Differentiated* | Reference | ||
| Undifferentiated† | 1.342 | 0.768–2.344 | 0.302 |
| Stage of esophageal cancer (TNM)‡ | |||
| I | Reference | ||
| II | 3.036 | 1.202–7.663 | 0.019 |
| III | 3.349 | 1.315–8.531 | 0.011 |
| IV | 8.026 | 2.972–21.675 | 0.000 |
| Stage of gastric cancer (TNM)‡ | |||
| I | Reference | ||
| II | 3.370 | 1.663–6.828 | 0.001 |
| III | 2.214 | 1.131–4.336 | 0.020 |
| IV | 128.885 | 7.880–2,108.098 | 0.001 |
| Treatment group, overall | <0.001 | ||
| Group 1 | Reference | ||
| Group 2 | 1.104 | 0.343–3.556 | 0.868 |
| Group 3 | 6.519 | 2.880–14.752 | <0.001 |
| Group 4 | 9.286 | 3.919–22.007 | <0.001 |
CI, confidence interval.
Differentiated: well or moderately differentiated;
Undifferentiated: poorly differentiated, undifferentiated, or signet ring cell;
Based on the American Joint Committee on Cancer 7th edition TNM system.
Table 5.
Multivariate Analysis of the Risk Factors for Death
| Hazard ratio | 95% CI | p-value | |
|---|---|---|---|
| Age | 1.076 | 1.033–1.120 | 0.000 |
| Treatment group, overall | 0.019 | ||
| Group 1 | Reference | ||
| Group 2 | 1.153 | 0.335–3.965 | 0.821 |
| Group 3 | 4.058 | 1.559–10.564 | 0.004 |
| Group 4 | 3.310 | 1.124–9.751 | 0.030 |
CI, confidence interval.
Fig. 1.
Kaplan-Meier estimates of the cumulative incidence of death in patients according to treatment group.
4. Comparison of morbidity and nutritional status
Clinical variables relating to complications and morbidity after treatment in group 1 and group 2 are compared in Table 6. The proportions of patients receiving intensive care (p=0.003), loss of body weight (p=0.042), and reduction in hemoglobin (p=0.033) at 24 months after treatment were lower in group 2 than in group 1 but there was no difference in death rate (48.1% vs 28.6%, p=0.228) or recurrence rate (22.2% vs 14.3%, p=0.354).
Table 6.
Comparison of Morbidity and Mortality between the Endoscopic and Surgical Treatment Groups
| Group 1 (n=27) | Group 2 (n=14) | p-value | |
|---|---|---|---|
| Death | 13 (48.1) | 4 (28.6) | 0.228 |
| Recurrence | 6 (22.2) | 2 (14.3) | 0.354 |
| ICU care | 17 (63.0) | 5 (35.7) | 0.003 |
| Body weight, baseline, kg | 60.70 | 60.99 | 0.924 |
| Δ12 mo–baseline | −7.403 | −7.286 | 0.971 |
| Δ24 mo–baseline | −11.743 | −6.140 | 0.042 |
| Albumin, baseline, g/dL | 3.70 | 3.82 | 0.420 |
| Δ12 mo–baseline | −0.109 | −0.020 | 0.706 |
| Δ24 mo–baseline | −0.408 | −0.014 | 0.167 |
| Hemoglobin, baseline, g/dL | 13.01 | 13.69 | 0.238 |
| Δ12 mo–baseline | −1.832 | −0.750 | 0.054 |
| Δ24 mo–baseline | −2.517 | −0.457 | 0.033 |
Data are presented as number of patients (%).
ICU, intensive care unit.
DISCUSSION
In the present study, we compared clinical outcomes according to treatment method in 79 patients with synchronous esophageal squamous cell carcinoma and gastric adenocarcinoma. We found that endoscopic resection for synchronous resectable esophageal and gastric cancer was feasible and safe, because survival was similar to that after surgical resection, and postoperative morbidity, especially the nutritional status of the patients, was lower than after surgery. Since the prognosis was significantly better when surgical and endoscopic resection was performed, early detection of synchronous esophageal and gastric cancer when they are resectable is desirable for good outcomes.
Although surgical resection for synchronous esophageal and gastric cancer may be adequate in terms of curability, the options are limited in advanced cases involving patients in poor clinical condition. Bai et al.4 reported that only 11 of 36 (30.6%) patients with synchronous esophageal and gastric cancer were able to undergo curative surgery while the rest received chemotherapy or palliative treatment because of distant metastases, severe comorbidities, and refusal of surgery. Recently, progress has been made in the endoscopic treatment of early gastric cancer and superficial esophageal cancer, and it has been suggested that endoscopic treatment is appropriate12 for completely curative and minimally invasive treatment of gastrointestinal mucosal cancer.13,14 Furthermore, it is advantageous to remove the gastric tumor endoscopically before surgery for esophageal cancer because the stomach can be used in the reconstruction.2 Until now, there has been only one report mentioning endoscopic resection for synchronous esophageal and gastric cancer.5 In the present study, 14 patients received endoscopic resection for one or both of their cancers with or without other treatments and their survival after surgical resection for both cancers or surgery for the gastric cancer with CRT for the esophageal cancer was similar.
After endoscopic resection there is a risk of metachronous esophageal or gastric cancer, as the field of carcinogenesis remains.15,16 Therefore, early detection of metachronous cancer through careful yearly endoscopic examination is considered to be needed for favorable long-term outcomes after endoscopic resection. Two patients in the present study were found to have metachronous esophageal cancers 8 and 20 months, respectively, after the original endoscopic resection for esophageal cancer, but both lesions were resected by endoscopic resection and both patients have been followed without further recurrence. Metachronous early gastric cancers were found in three patients who had received endoscopic resection for gastric cancer, and the lesions were removed by endoscopic or surgical resection in two instances, while the third patient refused further treatment. Despite the risk of metachronous cancer after endoscopic resection, which may reduce the cost effectiveness of this procedure,17 the superior quality of life and nutritional status that result could outweigh the disadvantages.
Although an association of esophageal squamous cell carcinoma with adenocarcinoma of the stomach has been reported sporadically in Japan,1,2 their synchronous occurrence is relatively uncommon.18 In this study, the frequency of synchronous esophageal and gastric cancer increased with time. In Korea, nationwide screening of men and women over 40 for gastric cancer started in 2002, and the proportion of the population recommended for screening increased annually by 4.2% between 2004 and 2011.19 We suggest that the increased frequency of synchronous esophageal and gastric cancer is due to improvements in endoscopy, the prolongation of life span, and the increase in upper endoscopic screening.
Nutritional status in patients with cancer is an important issue that influences quality of life, and malnutrition is an unavoidable consequence of surgical resection of esophageal and gastric cancers.20,21 The clinical parameters, such as change in body weight and hemoglobin, which are considered to reflect nutritional status,22,23 were more favorable after endoscopic resection than after surgical resection in the present study. Therefore, if endoscopic resection is possible for synchronous esophageal and gastric cancer, we suggest that it is superior to surgical resection with respect to nutritional status.
Our study has important limitations because its retrospective design meant that the treatments were not based on randomization. Although it is based on a large number of synchronous esophageal squamous cell carcinomas and gastric adenocarcinomas, it is still small for an analysis of the clinical variables. Nevertheless, its findings suggest an appropriate methodological design for future prospective randomized studies of the treatment of synchronous esophageal and gastric cancer.
In conclusion, since the prognosis is significantly better when surgical and endoscopic resection is performed, early detection of synchronous esophageal and gastric cancer when they are resectable is desirable. Endoscopic resection for synchronous resectable esophageal and gastric cancer is a possible alternative treatment method with similar rates of survival to surgical resection, and superior nutritional status after treatment.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Souquet JC, Berger F, Bonvoisin S, et al. Esophageal squamous cell carcinoma associated with gastric adenocarcinoma. Cancer. 1989;63:786–790. doi: 10.1002/1097-0142(19890215)63:4<786::AID-CNCR2820630430>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 2.Koide N, Komatsu D, Hiraga R, Kitazawa M, Suzuki A, Miyagawa S. Esophageal cancer associated with other primary cancers: historical comparison of clinicopathologic features in 359 esophageal cancer patients. Hepatogastroenterology. 2010;57:513–518. [PubMed] [Google Scholar]
- 3.Pasławski M, Złomaniec J, Rucińska E, Kołtyś W. Synchronous primary esophageal and gastric cancers. Ann Univ Mariae Curie Sklodowska Med. 2004;59:406–410. [PubMed] [Google Scholar]
- 4.Bai Y, Zou DW, Li ZS. Clinical presentation, endoscopic features, treatment and prognosis of synchronous upper gastrointestinal malignancies. J Dig Dis. 2012;13:19–23. doi: 10.1111/j.1751-2980.2011.00548.x. [DOI] [PubMed] [Google Scholar]
- 5.Ando N, Niwa Y, Ohmiya N, Ito B, Sasaki Y, Goto H. Simultaneous multiple early cancers of esophagus and stomach treated by endoscopic mucosal resection. Endoscopy. 2002;34:667–669. doi: 10.1055/s-2002-33240. [DOI] [PubMed] [Google Scholar]
- 6.Edge SB American Joint Committee on Cancer; American Cancer Society. AJCC cancer staging manual. 7th ed. New York: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 7.Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC. Pathology and genetics of tumours of the lung, pleura, thymus, and heart. Lyon: IARC Press; 2004. [Google Scholar]
- 8.Kim MK, Kim SB, Ahn JH, et al. Treatment outcome and recursive partitioning analysis-based prognostic factors in patients with esophageal squamous cell carcinoma receiving preoperative chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2008;71:725–734. doi: 10.1016/j.ijrobp.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi N, Isomoto H, Fukuda E, et al. Clinical outcomes of endoscopic submucosal dissection for early gastric cancer by indication criteria. Digestion. 2009;80:173–181. doi: 10.1159/000215388. [DOI] [PubMed] [Google Scholar]
- 10.Isomoto H, Shikuwa S, Yamaguchi N, et al. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58:331–336. doi: 10.1136/gut.2008.165381. [DOI] [PubMed] [Google Scholar]
- 11.Korea Ministry of Health and Welfare. Korea National Health and Nutrition Examination Survey [Internet] Cheongwon: Korea Center for Disease Control and Prevention; c2012. [cited 2012 Dec 26]. Available from: http://knhanes.cdc.go.kr. [Google Scholar]
- 12.Makuuchi H, Tanaka H, Shimada H, et al. Esophageal cancer and multiple primary cancer. Gan To Kagaku Ryoho. 1997;24:1–7. [PubMed] [Google Scholar]
- 13.Inoue H, Fukami N, Yoshida T, Kudo SE. Endoscopic mucosal resection for esophageal and gastric cancers. J Gastroenterol Hepatol. 2002;17:382–388. doi: 10.1046/j.1440-1746.2002.02732.x. [DOI] [PubMed] [Google Scholar]
- 14.Rembacken BJ, Gotoda T, Fujii T, Axon AT. Endoscopic mucosal resection. Endoscopy. 2001;33:709–718. doi: 10.1055/s-2001-16224. [DOI] [PubMed] [Google Scholar]
- 15.Correa P. Human gastric carcinogenesis: a multistep and multi-factorial process. First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 16.Katada C, Muto M, Manabe T, Ohtsu A, Yoshida S. Local recurrence of squamous-cell carcinoma of the esophagus after EMR. Gastrointest Endosc. 2005;61:219–225. doi: 10.1016/S0016-5107(04)02756-7. [DOI] [PubMed] [Google Scholar]
- 17.Choi KS, Jung HY, Choi KD, et al. EMR versus gastrectomy for intramucosal gastric cancer: comparison of long-term outcomes. Gastrointest Endosc. 2011;73:942–948. doi: 10.1016/j.gie.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 18.Goodner JT, Watson WL. Cancer of the esophagus: its association with other primary cancers. Cancer. 1956;9:1248–1252. doi: 10.1002/1097-0142(195611/12)9:6<1248::AID-CNCR2820090630>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 19.Park B, Choi KS, Lee YY, Jun JK, Seo HG. Trends in cancer screening rates among Korean men and women: results from the Korean National Cancer Screening Survey (KNCSS), 2004–2011. Cancer Res Treat. 2012;44:113–120. doi: 10.4143/crt.2012.44.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bae JM, Park JW, Yang HK, Kim JP. Nutritional status of gastric cancer patients after total gastrectomy. World J Surg. 1998;22:254–260. doi: 10.1007/s002689900379. [DOI] [PubMed] [Google Scholar]
- 21.Brain RH, Stammers FA. Sequelae of radical gastric resections: clinical and metabolic findings in 35 cases. Lancet. 1951;1:1137–1140. doi: 10.1016/S0140-6736(51)92654-2. [DOI] [PubMed] [Google Scholar]
- 22.Lis CG, Gupta D, Lammersfeld CA, Markman M, Vashi PG. Role of nutritional status in predicting quality of life outcomes in cancer: a systematic review of the epidemiological literature. Nutr J. 2012;11:27. doi: 10.1186/1475-2891-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta D, Lis CG, Granick J, Grutsch JF, Vashi PG, Lammersfeld CA. Malnutrition was associated with poor quality of life in colorectal cancer: a retrospective analysis. J Clin Epidemiol. 2006;59:704–709. doi: 10.1016/j.jclinepi.2005.08.020. [DOI] [PubMed] [Google Scholar]