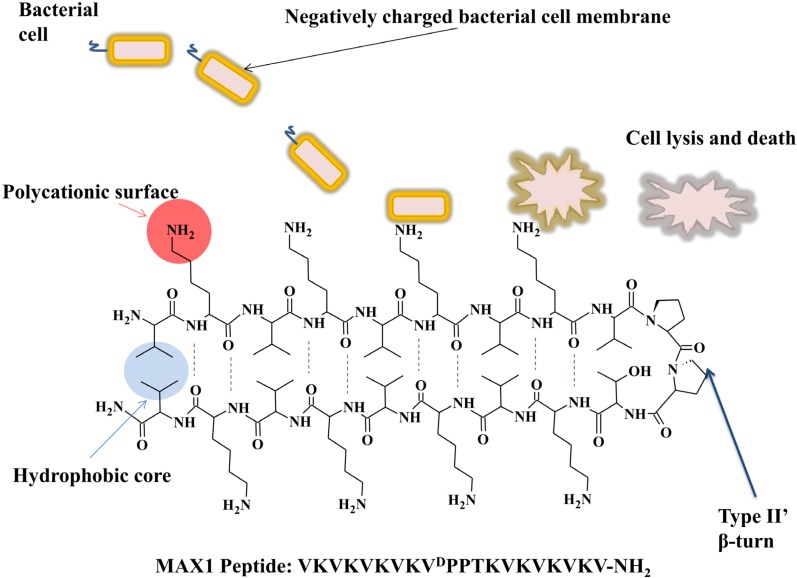
Figure 3.
The antibacterial mechanism of action of self-assembling β-sheet cationic peptides using the example of MAX1 peptide developed by the Schneider group [132]. Basic pH, above the pKa of lysine’s primary amine R-group (pH > 9), results in self-assembly of the primary peptide motif into a β-sheet secondary structure. The central VDPPT peptide forms a type II β-turn resulting in the formation of a hydrophobic valine core (blue) and a hydrophilic cationic lysine face (red). The primary amine (−NH2) R-groups of lysine protrude from the β-sheet structure forming a surface of polycationic character that is selective for negatively charged bacterial membranes. Adhesion and biofilm formation is prevented as bacterial membranes are compromised resulting in leakage of cell contents and bacterial cell death. In the hydrogel form the cationic groups may also displace divalent metal ions from the bacterial cell wall causing membrane disruption in biofilm cells, leading to cell death in both Gram-positive and –negative pathogens.