Abstract
Purpose
Dietary exposures during adolescence may exert important effects on breast development and future breast cancer risk. This study evaluated the associations between high school intakes of fat and micronutrients and the incidence of proliferative benign breast disease (BBD), a marker of increased breast cancer risk.
Methods
29,480 women (mean age: 43.3 years, range 33.6 to 52.9) completed a high school food frequency questionnaire (HS-FFQ) in 1998 in the Nurses’ Health Study II. Between 1991 and 2001, 682 women (follow-up time: 259,828 person years) were diagnosed with proliferative BBD whose biopsy slides were reviewed and confirmed by the study pathologists.
Results
In multivariate Cox proportional hazards models, high school intakes of fat and types of fat were not associated with proliferative BBD. Women in the highest quintile of total retinol activity equivalents (RAE), which incorporates retinol, alpha- and beta-carotene, and beta-cryptoxanthin intakes, had a 17% lower risk of proliferative BBD than those in the lowest quintile [multivariate hazard ratio (HR) [95% confidence interval (CI)]: 0.83 (0.64, 1.07), p-trend=0.01]; however, additional adjustment for high school dietary factors (vitamin D, nuts, and fiber) rendered the association non-significant [0.99 (0.73, 1.34), p-trend=0.32]. Results were similar with additional adjustment for adult RAE intake. Intakes of vitamin E and individual carotenoids were not associated with proliferative BBD, although an inverse association cannot be ruled out.
Conclusions
In this study, adolescent fat and micronutrient intakes were not associated with risk of proliferative BBD.
Keywords: fat, micronutrients, high school, dietary intake, proliferative benign breast disease
Introduction
Since the 1970s, when national breast cancer incidence rates were found to be strongly correlated with per capita fat consumption in each country [1], dietary fat intake has been suggested as a possible risk factor for breast cancer. Numerous studies conducted subsequently [2–5], particularly large cohort studies [4, 5] and clinical trials [6, 7], did not confirm the role of adult dietary fat in the breast carcinogenesis. More recently, certain micronutrients, such as vitamin A and vitamin E, have been hypothesized to be associated with a reduced risk of breast cancer due to their anti-oxidative properties [8, 9] and roles in cell proliferation, differentiation, apoptosis [10], and host immunology [11]. Results regarding the associations between these dietary factors and breast cancer risk, however, have been largely inconsistent [4, 12]. For carotenoids, the data are more convincing; recent meta-analyses and pooled analyses have noted an inverse association of both dietary [13, 14] and circulating [15, 16] carotenoids with breast cancer risk [e.g., for beta-carotene in a pooled analysis of 8 prospective cohort studies, women in the highest (vs. lowest) quintile of plasma/serum concentration for beta-carotene had a relative risk (RR)=0.83 [95% Confidence Interval(CI): 0.70, 1.01, p-trend=0.05] [15].
Benign breast disease (BBD) is a heterogeneous group of lesions that can develop during adolescence and young adulthood and includes a variety of histologic subtypes, among which proliferative BBD is a marker of subsequent breast cancer and may even be in the pathway for a subset of breast cancers [17]. Women whose biopsies show proliferative changes without atypia have a 1.3–1.9 fold greater risk of subsequent breast cancer than women with nonproliferative lesions, and women with atypical hyperplasia (AH) have a 3.9- to 13-fold greater risk [17]. Studies on diet and BBD could provide insights regarding the role of diet in the early stages of breast cancer development. Adult intake of total fat [18], animal fat [19], saturated fat, and polyunsaturated fat [20] was positively associated with BBD in some case-control studies. Only vegetable fat was inversely associated with BBD in the only prospective study conducted in the Nurses’ Health Study II (NHSII) cohort [21], and no association was found between adult fat intake and BBD in a case-cohort study in Canada [22]. One potential reason for the inconsistency across studies could be that a majority of the previous studies which observed an association between fat intake and BBD were case-control studies and retrospective in nature; thus, recall bias or selection bias was not completely avoidable and the observed association could be spurious. In addition, the lack of standard classification of BBD subtypes may result in misclassification and attenuate the associations. Furthermore, dietary intake during adulthood may not be an etiologically relevant exposure period.
Evidence from both animal [23] and human studies [24–26] suggests that exposures during childhood and adolescence may be important in breast cancer development, because breast tissue may be most vulnerable to carcinogens due to rapid proliferation of cells and lack of terminal differentiation during this time period. Higher vitamin E intake during adolescence was inversely associated with breast cancer risk in the Nurses’ Health Study (NHS) [27] and NHSII cohorts [28]. In a retrospective analysis in the NHSII in which women reported adolescent dietary intake after their diagnosis of BBD, high school intake of monounsaturated fat was positively associated with proliferative BBD risk, while vegetable fat and vitamin E had borderline significant inverse associations [29]. However, the retrospective nature of the study cannot completely rule out the possibility of differential recall for BBD cases and non-cases, and the small numbers of cases meant that power was limited to detect statistically significant associations.
We expanded upon the previous study [29] with the addition of more than 200 new cases (n = 682 total) and assessed the association between recalled high school diet and proliferative BBD. To the best of our knowledge, this is the first study to prospectively assess the associations between reported adolescent intakes of fats, carotenoids, and vitamins A and E and the incidence of proliferative BBD.
Materials and Methods
Study design and population
The NHSII cohort was established in 1989 when 116,671 U.S. female registered nurses aged between 25 and 42 years responded to a mailed, self-administered questionnaire asking about information on a variety of health-related exposures and conditions. The cohort has been followed up every two years by questionnaire since study initiation to obtain updated information on risk factors and disease outcomes, including diagnosis of BBD. The response rate in the NHSII cohort has been very high (≥90%) during each 2-year period questionnaire cycle [30]. In 1998, 45,948 women completed a semi-quantitative 131-item food frequency questionnaire (FFQ) asking about their usual dietary intake during adolescence, further defined as ages 13–18 years. High school diet questionnaire respondents had slightly larger childhood body size and were more likely to be nulliparous and have an older age at first birth than the non-respondents, but respondents and non-respondents were very similar in terms of other characteristics [31].
The current study included 45,948 women who returned the high school food frequency questionnaire in 1998 and had plausible values for total energy intake (between 600 and 5000 kcal/day). Women who had a previous self-reported or histologically confirmed diagnosis of BBD (n = 16,038), who reported a prior diagnosis of cancer other than non-melanoma skin cancer (n = 396), and whose biopsy date was before the return date of the 1991 questionnaire (n = 17), were excluded at 1991 baseline, leaving a total of 29,480 women.
High school food frequency questionnaire
The high school food frequency questionnaire (HS-FFQ) used in NHSII was a modified version of the well-validated adult diet FFQ used in the Nurses’ Health Study (NHS) and NHSII cohorts. However, it was designed to include commonly-consumed food items when this cohort of women would have been in high school, i.e., from 1960 to 1982. Foods that were not prevalent during that period of time were not included. Foods that account for major sources of fat, antioxidant vitamins, and carotenoids were included. The questionnaire asked participants how often, on average, they had consumed a specified unit or portion size of each food or beverage item during high school. Nine possible responses were provided ranging from ‘never or less than once per month’ to ‘six or more times per day’.
Nutrient intakes were computed for each participant by multiplying the frequency of consumption of each unit of food by the nutrient content of the specified portions and then summing the contributions from all foods, using an extensive food composition database derived using information from different sources and maintained by a team of research dietitians. Food composition data from the relevant time period (1960s and 1970s) were used, whenever available, as the composition of some foods has changed over time, to provide the best approximation of adolescent dietary intakes. Retinol activity equivalent (RAE) was calculated to represent vitamin A activity based on bioconversion from provitamin A carotenoids [32] using the formula: RAE (mcg) = (retinol, mcg) + (β-carotene from food, mcg / 12) + (β-carotene from vitamins and supplemented foods, mcg / 2) + (α-carotene, mcg / 24) + (β-cryptoxanthin, mcg / 24). This formula is based on the biochemical structures of provitamin A carotenoids and the number of provitamin A units needed for a given number of active vitamin A units.
The HS-FFQ is moderately reproducible [33], with intraclass correlations (ICC) ranging from 0.58 for polyunsaturated fat to 0.66 for animal and saturated fats, and from 0.61 for vitamin E without supplements to 0.72 for β-carotene. Recall of adolescent diet was not strongly affected by current diet, as the recalled adolescent diet was only weakly correlated with adult diet in 1995 (the last adult dietary assessment prior to the 1998 HS-FFQ) (for fat intakes, range = 0.07 for polyunsaturated fat to 0.24 for saturated fat; for micronutrients, range = −0.11 for vitamin E to 0.32 for vitamin A (retinol equivalents, RE) and β-carotene). When comparing the high school dietary data reported by the nurses themselves with reports of their high school diets by their mothers, Pearson correlations ranged from 0.30 for monounsaturated fat to 0.51 for animal and vegetable fatsand from 0.33 for β-carotene to 0.42 for vitamin A (RE) with or without supplements, suggesting that the HS-FFQ had modest validity.
Identification of BBD cases
Incident BBD cases were initially identified from the biennial follow-up questionnaires. Women who reported a first diagnosis of biopsy-confirmed BBD between 1993 and 2001 questionnaire cycles were contacted to seek diagnosis confirmation and to request permission to obtain their pathology specimens.
Between 1991 and 2001, a total of 3,273 women reported a first diagnosis of biopsy-confirmed BBD. Of these, 1,663 (50.8%) completed the HS-FFQ. Among the 1,662 (one woman was deleted due to missing all covariate information) women with adolescent diet information who were initially contacted, 1,333 (80.2%) confirmed the BBD diagnosis and gave permission to review their biopsy records and pathology slides. Pathology material was obtained and reviewed for 1,160 women (87.0% of those who had given their permission); and 1,149 women (99.1% of those for whom biopsy specimens were received) had valid biopsy information. The main reasons for exclusion included that the pathology specimen did not contain breast tissue or that the biopsy date was before 1989.
Three pathologists (LCC, SJS, JLC) without knowledge of participants’ adolescent diet information reviewed the collected benign breast biopsy slides independently and classified benign breast lesions into three broad categories of histology: nonproliferative, proliferative disease without atypia, and atypical hyperplasia (AH; ductal and lobular), using the criteria of Dupont and Page [34]. Any biopsy specimens that showed atypia or questionable atypia were jointly reviewed by two pathologists to reach a consensus diagnosis. Specimens with intraductal papilloma, radial scar, sclerosing adenosis, fibroadenoma, fibroadenomatous change, or moderate to florid ductal hyperplasia in the absence of AH were classified as proliferative without atypia. Proliferative BBD with or without atypia confirmed by pathology review was the outcome of interest in the current analysis, because proliferative BBD, in contrast with other histological subtypes, is associated with increased risk of breast cancer. Of the 1,149 women who had valid biopsy information, 717 (62.4%) were classified as proliferative (with or without atypia) by the study pathologists.
Statistical analysis
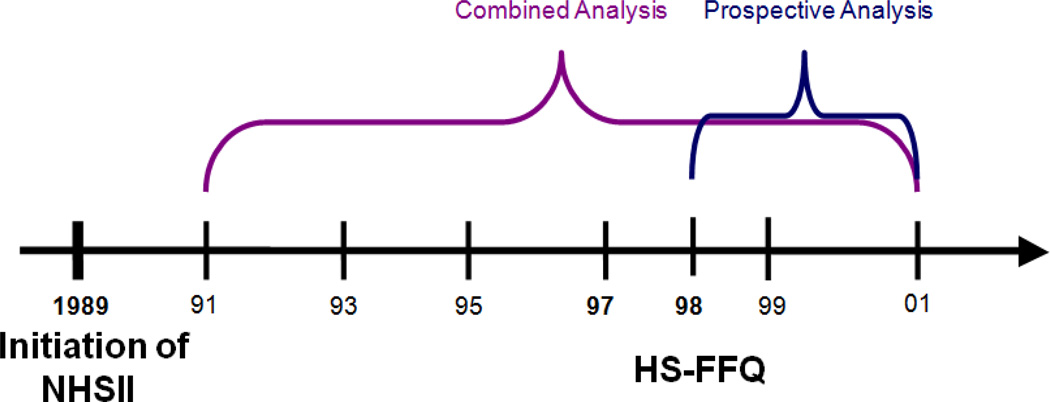
Our main analysis assessed high school diet in relation to all cases of proliferative BBD from 1991–2001 diagnosed before or after their return of the HS-FFQ (referred to as the combined analysis). To assess the possibility of recall bias, we conducted the analysis considering only proliferative BBD cases diagnosed after completion of the HS-FFQ (described hereafter as the prospective analysis). Because results from the prospective analysis and combined analysis were very similar and to increase study power, the combined analysis was considered as the primary analysis. In the combined analysis, each participant was followed up from the return date of the 1991 questionnaire until the return date of the 2001 questionnaire, death from any cause, BBD diagnosis, report of cancer other than non-melanoma skin cancer, or loss to follow-up, whichever came first. In the prospective analysis, follow-up started from the return date of the 1998 high school diet questionnaire. A total of 23,946 women were included in the prospective analysis. After the baseline exclusions in 1991 or 1998, the total numbers of cases in the final combined and prospective analyses were 682 and 142, respectively. Atypical hyperplasia was not examined as a separate outcome due to the small numbers of cases (n = 61 and 14, respectively).
Total fat, types of fat, and a variety of micronutrients were the main exposures of interest in the current study. To adjust for total energy intake [35], we used nutrient densities, computed as percentages of total caloric intake, for total fat and types of fat. For micronutrients, the residual method was used, in which energy-adjusted values are the residuals from a regression model with total caloric intake as the independent variable and absolute micronutrient intake as the dependent variable [35]. We divided fat densities and energy-adjusted nutrient residuals into quintiles based on the distribution of intakes for all eligible women.
To estimate the associations between nutrient intakes and the risk of proliferative BBD, multivariable-adjusted Cox proportional hazards regression was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs), using the lowest quintile as the reference group and follow-up time as the time variable. The final models were adjusted for age in months, total energy intake, and other potential confounders, including age at menarche, menopausal status, average body size between ages 5 and 10 years, family history of breast cancer in mother or sister(s), alcohol intake between ages 18 and 22 years, multivitamin use between ages 13 and 18 years, recency and duration of oral contraceptive use, and parity and age at first birth. The time-varying covariates of age, menopausal status, oral contraceptive use, and parity and age at first birth were updated every two years in each questionnaire cycle. Family history of breast cancer was initially assessed in 1989 and updated in 1997. In our final model, we additionally adjusted for adolescent intakes of vitamin D, fiber, and nuts, dietary variables associated with BBD in this cohort [31, 36]. In the models where type of fat was the exposure variable, we also adjusted for the other types of fat in the final model. For instance, when saturated fat was the exposure of interest, we additionally adjusted for monounsaturated, polyunsaturated, and trans-unsaturated fats.
SAS PROC PHREG was used for all analyses, and the Anderson-Gill data structure [37] was used to handle time-varying covariates efficiently, with a new data record created for every questionnaire cycle at which a participant was at risk and covariates set to their values at the time of return of questionnaire. To test for trend, the Wald statistic was calculated by including the median value of each quintile as a continuous variable in the model.
The study was approved by human research committees at the Harvard School of Public Health and Brigham and Women’s Hospital.
Results
Table 1 presents the baseline distributions in 1991 of selected characteristics of participants, according to their intakes of total fat, RAE, and vitamin E during adolescence. Compared to women with low dietary fat intake during adolescence, women with high intake of total fat were more likely to have a larger body size between ages 5 and 10 and higher alcohol consumption between ages 18 and 22, and to have ever used oral contraceptives, but less likely to have used multivitamins in high school. Women with high intakes of micronutrients were more likely to be nulliparous, have an older age at first birth, and much more likely to have used multivitamins in high school.
Table 1.
Age-standardized characteristics of participants according to total fat, retinol activity equivalents (RAE), and vitamin E intake during adolescence among 29,480 women in the Nurses’ Health Study II.a
| Total fatb | Total retinol activity equivalents (mcg/day)c |
Total vitamin E (mg/day)c |
||||
|---|---|---|---|---|---|---|
| Quintile 1 (low) |
Quintile 5 (high) |
Quintile 1 (low) |
Quintile 5 (high) |
Quintile 1 (low) |
Quintile 5 (high) |
|
| Number of women Percentage (%) | 5,896 | 5,896 | 5,897 | 5,891 | 5,927 | 5,889 |
| Family history of breast cancer in mother or sister(s) | 5 | 5 | 5 | 5 | 4 | 5 |
| Age at menarche < 12 yrs | 25 | 25 | 23 | 25 | 22 | 26 |
| Premenopausal | 98 | 98 | 98 | 98 | 98 | 98 |
| Average body size between ages 5 and 10 years, somatotype pictogram category >3d | 25 | 31 | 27 | 26 | 24 | 31 |
| Nulliparous | 29 | 27 | 25 | 31 | 26 | 30 |
| Ever oral contraceptive use | 82 | 87 | 87 | 83 | 84 | 85 |
| Multivitamin use between ages 13 and 18 | 21 | 10 | 0 | 60 | 11 | 24 |
| Mean | ||||||
| Age (yrs) | 34.3 | 37.2 | 35.8 | 35.7 | 36.7 | 34.6 |
| Alcohol intake between ages 18 and 22 (g/day) | 4.4 | 5.7 | 5.3 | 4.8 | 4.9 | 5.4 |
| Paritye | 2.1 | 2.1 | 2.2 | 2.1 | 2.2 | 2.1 |
| Age at first birthe (yrs) | 26.1 | 25.7 | 25.4 | 26.2 | 25.7 | 26.1 |
Except for the data on mean age, all data shown are standardized to the age distribution of the cohort in 1991. Except for multivitamin use between ages 13 and 18, all data shown are 1991 baseline values.
Quintile of percent calories from fat.
Quintile of energy-adjusted nutrient residuals.
Based on a 9 point pictogram scale. (Stunkard et al.)
Among parous women.
No association was observed between adolescent total fat intake and proliferative BBD risk (Table 2). Similarly, we found no associations for high school intakes of different types of fat: animal fat, vegetable fat, saturated fat, monounsaturated fat, polyunsaturated fat, and trans-unsaturated fat. Additionally, total fat and types of fat were not associated with proliferative BBD in the prospective analysis.
Table 2.
Percentage of calories from total fat and types of fat during adolescence and relative risks (95% confidence interval) of proliferative benign breast disease in NHSII.
| Exposures | Quintiles of percentage of calories from fat | P trend | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Total fat | ||||||
| Median intake (% of energy) | 34.8 | 38.4 | 40.7 | 42.8 | 46.0 | |
| No. of combined BBD casesa | 126 | 144 | 120 | 138 | 154 | |
| Multivariate RR (95% CI)b | 1.00 (ref) | 1.08 (0.85, 1.38) | 0.88 (0.69, 1.14) | 0.98 (0.77, 1.26) | 1.09 (0.85, 1.39) | 0.72 |
| Additional adjustment for fiber, nuts, and vitamin D | 1.00 (ref) | 1.04 (0.81, 1.32) | 0.82 (0.63, 1.06) | 0.89 (0.68, 1.15) | 0.96 (0.73, 1.25) | 0.50 |
| No. of incident BBD casesc | 25 | 31 | 28 | 29 | 29 | |
| Multivariate RR (95% CI)d | 1.00 (ref) | 1.25 (0.73, 2.14) | 1.13 (0.65, 1.96) | 1.21 (0.70, 2.10) | 1.26 (0.72, 2.19) | 0.49 |
| Additional adjustment for fiber, nuts, and vitamin D | 1.00 (ref) | 1.18 (0.69, 2.04) | 1.02 (0.57, 1.81) | 1.04 (0.58, 1.87) | 0.99 (0.54, 1.83) | 0.86 |
| Animal fat | ||||||
| Median intake (% of energy) | 18.6 | 22.6 | 25.4 | 28.5 | 32.9 | |
| No. of combined BBD casesa | 112 | 121 | 141 | 151 | 157 | |
| Multivariate RR (95% CI)b | 1.00 (ref) | 1.02 (0.78, 1.32) | 1.10 (0.84, 1.42) | 1.15 (0.89, 1.50) | 1.15 (0.88, 1.51) | 0.20 |
| Additional adjustment for vegetable fatb and fiber, nuts, and vitamin D | 1.00 (ref) | 0.96 (0.73, 1.25) | 0.98 (0.74, 1.30) | 0.99 (0.73, 1.33) | 0.95 (0.68, 1.32) | 0.82 |
| No. of incident BBD casesc | 26 | 29 | 34 | 25 | 28 | |
| Multivariate RR (95% CI)d | 1.00 (ref) | 1.25 (0.72, 2.16) | 1.47 (0.85, 2.53) | 1.01 (0.56, 1.83) | 1.19 (0.66, 2.17) | 0.83 |
| Additional adjustment for vegetable fatd and fiber, nuts, and vitamin D | 1.00 (ref) | 1.17 (0.67, 2.05) | 1.28 (0.71, 2.32) | 0.79 (0.40, 1.55) | 0.87 (0.41, 1.85) | 0.48 |
| Vegetable fat | ||||||
| Median intake (% of energy) | 9.8 | 12.6 | 14.7 | 16.8 | 20.0 | |
| No. of combined BBD casesa | 158 | 140 | 147 | 126 | 111 | |
| Multivariate RR (95% CI)b | 1.00 (ref) | 0.93 (0.74, 1.17) | 1.01 (0.80, 1.28) | 0.88 (0.69, 1.13) | 0.82 (0.63, 1.07) | 0.15 |
| Additional adjustment for animal fatb and fiber, nuts, and vitamin D | 1.00 (ref) | 0.92 (0.72, 1.17) | 0.99 (0.77, 1.28) | 0.86 (0.66, 1.13) | 0.80 (0.59, 1.09) | 0.15 |
| No. of incident BBD casesc | 30 | 26 | 35 | 22 | 29 | |
| Multivariate RR (95% CI)d | 1.00 (ref) | 0.92 (0.54, 1.56) | 1.31 (0.78, 2.17) | 0.80 (0.45, 1.42) | 1.03 (0.59, 1.81) | 0.95 |
| Additional adjustment for animal fatd and fiber, nuts, and vitamin D | 1.00 (ref) | 0.86 (0.49,1.50) | 1.18 (0.67, 2.07) | 0.69 (0.36, 1.32) | 0.94 (0.48, 1.82) | 0.69 |
| Saturated fat | ||||||
| Median intake (% of energy) | 12.9 | 14.6 | 15.9 | 17.2 | 19.2 | |
| No. of combined BBD casesa | 115 | 141 | 132 | 138 | 156 | |
| Multivariate RR (95% CI)b | 1.00 (ref) | 1.18 (0.92, 1.51) | 1.04 (0.81, 1.35) | 1.04 (0.80, 1.35) | 1.13 (0.87, 1.46) | 0.62 |
| Additional adjustment for other types of fatb and fiber, nuts, and vitamin D | 1.00 (ref) | 1.13 (0.86, 1.48) | 0.95 (0.70, 1.28) | 0.91 (0.65, 1.27) | 0.93 (0.64, 1.36) | 0.48 |
| No. of incident BBD casesc | 28 | 27 | 30 | 28 | 29 | |
| Multivariate RR (95% CI)d | 1.00 (ref) | 1.00 (0.58, 1.72) | 1.13 (0.66, 1.93) | 1.04 (0.60, 1.81) | 1.10 (0.62, 1.92) | 0.74 |
| Additional adjustment for other types of fatd and fiber, nuts, and vitamin D | 1.00 (ref) | 0.94 (0.52, 1.68) | 1.03 (0.54, 1.94) | 0.90 (0.44, 1.81) | 0.94 (0.41, 2.15) | 0.87 |
| Monounsaturated fat | ||||||
| Median intake (% of energy) | 12.3 | 13.7 | 14.6 | 15.4 | 16.7 | |
| No. of combined BBD casesa | 129 | 129 | 147 | 127 | 150 | |
| Multivariate RR (95% CI)b | 1.00 (ref) | 0.97 (0.75, 1.23) | 1.12 (0.88, 1.42) | 0.94 (0.73, 1.20) | 1.10 (0.86, 1.39) | 0.55 |
| Additional adjustment for other types of fatb and fiber, nuts, and vitamin D | 1.00 (ref) | 0.95 (0.72, 1.25) | 1.12 (0.83, 1.50) | 0.93 (0.67, 1.29) | 1.09 (0.76, 1.57) | 0.67 |
| No. of incident BBD casesc | 26 | 32 | 31 | 25 | 28 | |
| Multivariate RR (95% CI)d | 1.00 (ref) | 1.24 (0.73, 2.10) | 1.26 (0.74, 2.14) | 1.00 (0.57, 1.74) | 1.13 (0.66, 1.96) | 0.90 |
| Additional adjustment for other types of fatd and fiber, nuts, and vitamin D | 1.00 (ref) | 1.20 (0.67, 2.15) | 1.16 (0.61, 2.23) | 0.88 (0.43, 1.79) | 0.90 (0.41, 1.99) | 0.56 |
| Polyunsaturated fat | ||||||
| Median intake (% of energy) | 5.0 | 5.9 | 6.5 | 7.2 | 8.3 | |
| No. of combined BBD casesa | 152 | 135 | 145 | 134 | 116 | |
| Multivariate RR (95% CI)b | 1.00 (ref) | 0.91 (0.72, 1.15) | 1.01 (0.80, 1.27) | 0.94 (0.74, 1.19) | 0.82 (0.64, 1.05) | 0.16 |
| Additional adjustment for other types of fatb and fiber, nuts, and vitamin D | 1.00 (ref) | 0.90 (0.71, 1.14) | 0.99 (0.78, 1.27) | 0.92 (0.71, 1.20) | 0.81 (0.61, 1.07) | 0.19 |
| No. of incident BBD casesc | 30 | 29 | 32 | 22 | 29 | |
| Multivariate RR (95% CI)d | 1.00 (ref) | 0.98 (0.58, 1.65) | 1.04 (0.63, 1.73) | 0.77 (0.44, 1.35) | 1.04 (0.61, 1.75) | 0.88 |
| Additional adjustment for other types of fatd and fiber, nuts, and vitamin D | 1.00 (ref) | 1.02 (0.60, 1.73) | 1.12 (0.65, 1.92) | 0.87 (0.47, 1.59) | 1.20 (0.66, 2.19) | 0.69 |
| Trans-unsaturated fat | ||||||
| Median intake (% of energy) | 1.5 | 1.9 | 2.3 | 2.7 | 3.3 | |
| No. of combined BBD casesa | 139 | 147 | 130 | 142 | 124 | |
| Multivariate RR (95% CI)b | 1.00 (ref) | 1.15 (0.91, 1.46) | 1.07 (0.84, 1.36) | 1.16 (0.91, 1.48) | 0.98 (0.77, 1.26) | 0.84 |
| Additional adjustment for other types of fatb and fiber, nuts, and vitamin D | 1.00 (ref) | 1.13 (0.89, 1.44) | 1.03 (0.80, 1.33) | 1.11 (0.86, 1.43) | 0.92 (0.70, 1.20) | 0.43 |
| No. of incident BBD casesc | 34 | 27 | 22 | 33 | 26 | |
| Multivariate RR (95% CI)d | 1.00 (ref) | 0.84 (0.50, 1.40) | 0.70 (0.40, 1.23) | 1.11 (0.67, 1.83) | 0.84 (0.49, 1.42) | 0.85 |
| Additional adjustment for other types of fatd and fiber, nuts, and vitamin D | 1.00 (ref) | 0.77 (0.46, 1.30) | 0.61 (0.34, 1.09) | 0.94 (0.55, 1.62) | 0.67 (0.38, 1.21) | 0.37 |
682 cases of proliferative BBD (with or without atypia) were diagnosed during the follow-up period 1991–2001.
The multivariate models are adjusted for age in months, time period (5 periods), total energy intake (quintiles), age at menarche (<12, 12, 13, or ≥ 14 years), menopausal status (premenopausal, postmenopausal, or uncertain), average body size between ages 5 and 10 (somatotype pictogram 1, 1.5–2, 2.5–3, 3.5–4.5, or ≥ 5), family history of breast cancer in mother or sister (yes vs. no), alcohol intake between ages 18 and 22 years (0, <5, 5–14, or ≥ 15 grams/day), multivitamin use between ages 13 and 18 years (yes vs. no), recency and duration of OC use (never, past <4 years, past ≥ 4 years, current <4 years, or current ≥4 years), and parity and age at first birth (nulliparous, 1–2 pregnancies and age at first birth <25 years, 1–2 pregnancies and age at first birth 25–29 years, 1–2 pregnancies and age at first birth ≥ 30 years, ≥ 3 pregnancies and age at first birth <25 years, ≥ 3 pregnancies and age at first birth 25–29 years, or ≥3 pregnancies and age at first birth ≥ 30 years). Age-adjusted results were similar.
142 cases of proliferative BBD (with or without atypia) were diagnosed during the follow-up period 1998–2001.
The multivariate models are adjusted for variables categorized as above, except for time period (2 periods), recency and duration of OC use (never, past <4 years, past ≥ 4 years, or current), and parity and age at first birth (nulliparous, 1–2 pregnancies and age at first birth <25 years, 1–2 pregnancies and age at first birth 25–29 years, 1–2 pregnancies and age at first birth ≥ 30 years, ≥ 3 pregnancies and age at first birth <25 years, ≥ 3 pregnancies and age at first birth ≥ 25 years). Age-adjusted results were similar.
Overall, adolescent intakes of the micronutrients examined were not associated with BBD risk in the combined analysis or the prospective analysis (Table 3). Women in the highest quintile of RAE had a slightly lower risk of proliferative BBD than those in the lowest quintile [multivariate hazard ratio (HR) (95% CI)]: 0.83 (0.64, 1.07), p-trend=0.01]. Results were similar with additional adjustment for adult RAE intake. However, additional adjustment for high school dietary factors (vitamin D, nuts, and fiber) rendered the association non-significant (0.99 (0.73, 1.34), p-trend=0.32]. In sum, intakes of vitamin E and individual carotenoids were not associated with proliferative BBD, although an inverse association cannot be ruled out.
Table 3.
Energy-adjusted micronutrient intake during adolescence and relative risk (95% confidence interval) of proliferative benign breast disease in NHSII.a
| Exposures | Quintile of energy-adjusted micronutrient intake | P trend |
||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Retinol activity equivalents (RAE) | ||||||
| Median intake (mcg/day) | 710 | 913 | 1100 | 1370 | 2179 | |
| No. of combined BBD casesb | 133 | 155 | 164 | 124 | 106 | |
| Multivariate RR (95% CI) | 1.00 (ref) | 1.19 (0.94, 1.50) | 1.27 (1.01, 1.60) | 0.98 (0.76, 1.25) | 0.83 (0.64, 1.07) | 0.01 |
| Additional adjustment for fiber, nuts, and vitamin D | 1.00 (ref) | 1.24 (0.98, 1.58) | 1.37 (1.08, 1.75) | 1.09 (0.84, 1.43) | 0.99 (0.73, 1.34) | 0.32 |
| No. of incident BBD casesc | 31 | 33 | 31 | 25 | 22 | |
| Multivariate RR (95% CI) | 1.00 (ref) | 1.06 (0.65, 1.75) | 1.01 (0.61, 1.68) | 0.77 (0.45, 1.32) | 0.73 (0.42, 1.28) | 0.14 |
| Additional adjustment for fiber, nuts, and vitamin D | 1.00 (ref) | 1.11 (0.67, 1.86) | 1.11 (0.65, 1.89) | 0.88 (0.49, 1.58) | 0.93 (0.48, 1.81) | 0.63 |
| RAE from food only | ||||||
| Median intake (mcg/day) | 699 | 884 | 1041 | 1236 | 1638 | |
| No. of combined BBD casesb | 135 | 142 | 155 | 130 | 120 | |
| Multivariate RR (95% CI) | 1.00 (ref) | 1.08 (0.85, 1.37) | 1.20 (0.95, 1.51) | 1.02 (0.80, 1.30) | 0.94 (0.73, 1.20) | 0.37 |
| Additional adjustment for fiber, nuts, and vitamin D | 1.00 (ref) | 1.15 (0.90, 1.46) | 1.31 (1.03, 1.67) | 1.17 (0.90, 1.51) | 1.12 (0.86, 1.47) | 0.60 |
| No. of incident BBD casesc | 29 | 34 | 31 | 26 | 22 | |
| Multivariate RR (95% CI) | 1.00 (ref) | 1.16 (0.70, 1.91) | 1.11 (0.66, 1.85) | 0.88 (0.51, 1.51) | 0.70 (0.39, 1.24) | 0.10 |
| Additional adjustment for fiber, nuts, and vitamin D | 1.00 (ref) | 1.24 (0.74, 2.07) | 1.26 (0.74, 2.15) | 1.06 (0.60, 1.87) | 0.88 (0.47, 1.63) | 0.46 |
| Vitamin E | ||||||
| Median intake (mg/day) | 9.8 | 11.3 | 12.4 | 13.6 | 15.7 | |
| No. of combined BBD casesb | 147 | 160 | 140 | 112 | 123 | |
| Multivariate RR (95% CI) | 1.00 (ref) | 1.14 (0.91, 1.43) | 1.00 (0.79, 1.27) | 0.82 (0.64, 1.05) | 0.90 (0.70, 1.15) | 0.08 |
| Additional adjustment for fiber, nuts, and vitamin D | 1.00 (ref) | 1.16 (0.93, 1.46) | 1.04 (0.82, 1.32) | 0.86 (0.67, 1.11) | 0.97 (0.76, 1.25) | 0.30 |
| No. of incident BBD casesc | 36 | 28 | 29 | 19 | 30 | |
| Multivariate RR (95% CI) | 1.00 (ref) | 0.80 (0.49, 1.32) | 0.86 (0.52, 1.43) | 0.54 (0.31, 0.95) | 0.87 (0.52, 1.43) | 0.37 |
| Additional adjustment for fiber, nuts, and vitamin D | 1.00 (ref) | 0.83 (0.50, 1.38) | 0.94 (0.56, 1.56) | 0.61 (0.34, 1.08) | 1.02 (0.60, 1.72) | 0.83 |
| Vitamin E from food only | ||||||
| Median intake (mg/day) | 9.8 | 11.3 | 12.3 | 13.4 | 15.3 | |
| No. of combined BBD casesb | 145 | 159 | 145 | 110 | 123 | |
| Multivariate RR (95% CI) | 1.00 (ref) | 1.15 (0.91, 1.44) | 1.05 (0.83, 1.33) | 0.81 (0.63, 1.04) | 0.87 (0.68, 1.12) | 0.04 |
| Additional adjustment for fiber, nuts, and vitaminD | 1.00 (ref) | 1.16 (0.92, 1.46) | 1.08 (0.85, 1.37) | 0.84 (0.65, 1.09) | 0.92 (0.71, 1.18) | 0.13 |
| No. of incident BBD casesc | 36 | 32 | 28 | 19 | 27 | |
| Multivariate RR (95% CI) | 1.00 (ref) | 0.91 (0.56, 1.47) | 0.78 (0.47, 1.29) | 0.55 (0.31, 0.96) | 0.77 (0.46, 1.28) | 0.13 |
| Additional adjustment for fiber, nuts, and vitamin D | 1.00 (ref) | 0.94 (0.58, 1.53) | 0.84 (0.50, 1.40) | 0.60 (0.33, 1.07) | 0.84 (0.49, 1.43) | 0.28 |
| α-Carotene | ||||||
| Median intake (mcg/day) | 310 | 598 | 856 | 1283 | 2295 | |
| No. of combined BBD casesb | 139 | 146 | 129 | 142 | 126 | |
| Multivariate RR (95% CI) | 1.00 (ref) | 1.02 (0.81, 1.29) | 0.92 (0.72, 1.17) | 1.00 (0.79, 1.26) | 0.90 (0.71, 1.15) | 0.39 |
| Additional adjustment for fiber, nuts, and vitamin D | 1.00 (ref) | 1.07 (0.84, 1.35) | 1.01 (0.78, 1.29) | 1.13 (0.87, 1.45) | 1.08 (0.82, 1.43) | 0.56 |
| No. of incident BBD casesc | 32 | 30 | 25 | 30 | 25 | |
| Multivariate RR (95% CI) | 1.00 (ref) | 0.96 (0.58, 1.58) | 0.76 (0.44, 1.30) | 0.94 (0.56, 1.56) | 0.78 (0.46, 1.33) | 0.42 |
| Additional adjustment for fiber, nuts, and vitamin D | 1.00 (ref) | 1.03 (0.62, 1.73) | 0.90 (0.51, 1.57) | 1.14 (0.66, 1.96) | 1.02 (0.55, 1.88) | 0.85 |
| β-Carotene | ||||||
| Median intake (mcg/day) | 1599 | 2509 | 3319 | 4411 | 6713 | |
| No. of combined BBD casesb | 146 | 123 | 147 | 144 | 122 | |
| Multivariate RR (95% CI) | 1.00 (ref) | 0.85 (0.67, 1.08) | 1.01 (0.80, 1.27) | 0.98 (0.78, 1.24) | 0.85 (0.66, 1.08) | 0.35 |
| Additional adjustment for fiber, nuts, and vitamin D | 1.00 (ref) | 0.90 (0.70, 1.15) | 1.13 (0.88, 1.44) | 1.16 (0.89, 1.51) | 1.09 (0.81, 1.47) | 0.33 |
| No. of incident BBD casesc | 30 | 27 | 38 | 25 | 22 | |
| Multivariate RR (95% CI) | 1.00 (ref) | 0.91 (0.54, 1.54) | 1.27 (0.78, 2.07) | 0.82 (0.48, 1.42) | 0.72 (0.41, 1.26) | 0.17 |
| Additional adjustment for fiber, nuts, and vitamin D | 1.00 (ref) | 1.01 (0.59, 1.72) | 1.52 (0.90, 2.57) | 1.07 (0.58, 1.95) | 0.97 (0.49, 1.93) | 0.85 |
| β-Cryptoxanthin | ||||||
| Median intake (mcg/day) | 57 | 103 | 154 | 212 | 304 | |
| No. of combined BBD casesb | 135 | 119 | 159 | 127 | 142 | |
| Multivariate RR (95% CI) | 1.00 (ref) | 0.88 (0.69, 1.13) | 1.19 (0.95, 1.51) | 0.93 (0.73, 1.19) | 1.06 (0.83, 1.34) | 0.57 |
| Additional adjustment for fiber, nuts, and vitamin D | 1.00 (ref) | 0.92 (0.72, 1.19) | 1.29 (1.01, 1.63) | 1.04 (0.81, 1.34) | 1.22 (0.94, 1.57) | 0.09 |
| No. of incident BBD casesc | 31 | 16 | 35 | 31 | 29 | |
| Multivariate RR (95% CI) | 1.00 (ref) | 0.53 (0.29, 0.97) | 1.13 (0.69, 1.84) | 0.98 (0.59, 1.64) | 0.88 (0.53, 1.48) | 0.74 |
| Additional adjustment for fiber, nuts, and vitamin D | 1.00 (ref) | 0.58 (0.31, 1.07) | 1.32 (0.79, 2.18) | 1.19 (0.70, 2.02) | 1.12 (0.64, 1.95) | 0.26 |
| Lycopene | ||||||
| Median intake (mcg/day) | 3374 | 4770 | 5975 | 8359 | 12803 | |
| No. of combined BBD casesb | 135 | 138 | 149 | 141 | 119 | |
| Multivariate RR (95% CI) | 1.00 (ref) | 1.08 (0.85, 1.38) | 1.16 (0.91, 1.47) | 1.09 (0.86, 1.38) | 0.95 (0.74, 1.22) | 0.46 |
| Additional adjustment for fiber, nuts, and vitamin D | 1.00 (ref) | 1.09 (0.86, 1.39) | 1.18 (0.93, 1.50) | 1.12 (0.88, 1.43) | 0.99 (0.77, 1.29) | 0.71 |
| No. of incident BBD casesc | 29 | 32 | 29 | 24 | 28 | |
| Multivariate RR (95% CI) | 1.00 (ref) | 1.10 (0.66, 1.83) | 1.01 (0.60, 1.71) | 0.82 (0.47, 1.43) | 1.00 (0.59, 1.70) | 0.74 |
| Additional adjustment for fiber, nuts, and vitamin D | 1.00 (ref) | 1.11 (0.67, 1.86) | 1.06 (0.62, 1.81) | 0.88 (0.50, 1.53) | 1.11 (0.64, 1.93) | 0.93 |
| Lutein & zeaxanthin | ||||||
| Median intake (mcg/day) | 938 | 1473 | 1997 | 2658 | 4144 | |
| No. of combined BBD casesb | 140 | 139 | 132 | 145 | 126 | |
| Multivariate RR (95% CI) | 1.00 (ref) | 0.99 (0.78, 1.25) | 0.95 (0.75, 1.21) | 1.02 (0.81, 1.30) | 0.90 (0.71, 1.15) | 0.46 |
| Additional adjustment for fiber, nuts, and vitamin D | 1.00 (ref) | 1.05 (0.82, 1.34) | 1.05 (0.81, 1.36) | 1.19 (0.92, 1.54) | 1.12 (0.84, 1.49) | 0.37 |
| No. of incident BBD casesc | 28 | 29 | 30 | 31 | 24 | |
| Multivariate RR (95% CI) | 1.00 (ref) | 1.04 (0.62, 1.76) | 1.07 (0.64, 1.80) | 1.08 (0.64, 1.82) | 0.79 (0.45, 1.38) | 0.37 |
| Additional adjustment for fiber, nuts, and vitamin D | 1.00 (ref) | 1.19 (0.69, 2.04) | 1.31 (0.75, 2.29) | 1.42 (0.80, 2.53) | 1.13 (0.59, 2.17) | 0.77 |
The multivariate models were adjusted for the same variables as in the combined and incident BBD analyses in Table 2. Age-adjusted results were similar. Models of total micronutrient intake (including supplements) not adjusted for multivitamin intake. Variables represent total intake unless otherwise stated.
682 cases of proliferative BBD (with or without atypia) were diagnosed during the follow-up period 1991–2001.
142 cases of proliferative BBD (with or without atypia) were diagnosed during the follow-up period 1998–2001.
Discussion
In this large cohort study, we did not observe associations of adolescent intake of dietary fats with proliferative BBD. There was a suggestive inverse association between adolescent RAE and proliferative BBD risk, but this association became null when further adjusted for other adolescent dietary risk factors for BBD. Adolescent intakes of carotenoids and vitamin E were not associated with proliferative BBD in the combined analysis or the prospective analysis, although an inverse association cannot be ruled out.
Our null results are inconsistent with some of the results of our previous retrospective study of adolescent diet and proliferative BBD risk in this population [29], findings on adolescent diet and BBD in the Growing Up Today Study [38, 39], as well as results of adolescent diet and breast cancer in the NHS and NHSII [27, 28]. In these previous studies, adolescent vegetable fat, carotenoids, and vitamin E intakes were associated with a reduced risk of BBD and breast cancer [27–29, 38, 39]. However, the previous associations with BBD were only borderline significant [e.g., highest vs. lowest quartile of vegetable fat: RR=0.77 (95% CI: 0.56, 1.06, p-trend=0.15); vitamin E: RR=0.79 (95% CI: 0.61, 1.04, p-trend=0.05) in final models] [29], and in the breast cancer analysis by Frazier et al. (2004), the association with vegetable fat was no longer significant after adjusting for vitamin E, suggesting that the observed associations in those analyses may have been attributable to vitamin E or other dietary factors [28]. Monounsaturated fat was positively associated with BBD in the previous study [highest vs. lowest quartile RR=1.52 (95% CI: 1.06, 2.21, p-trend=0.03)] [29], whereas we did not observe an association in this larger study.
More recently in meta-analyses and pooled analyses, data from prospective studies show a more consistent and inverse association of both dietary [13, 14] and circulating [15, 16] carotenoids and breast cancer risk. In addition, the associations between antioxidants and breast cancer may be potentially modified by oxidative stress. For instance, high intake of vitamin C or β-carotene was associated with a reduced breast cancer risk in postmenopausal women taking exogenous hormones and for β-carotene consuming moderate to high amounts of alcohol in the EPIC studies [40].
The discrepancies in our findings in this analysis from previous studies in this cohort could be due to several factors. First, the previous BBD analysis in NHSII had a small number of cases and suggestive associations, but with a larger sample size, the associations did not hold up; perhaps the suggestive associations observed earlier occurred by chance and do not represent true associations. The previous study could have been affected by recall bias, whereas in this study the additional cases were prospective. The previous analysis did not adjust for vitamin D, nuts, or fiber, variables more recently found to be associated with BBD risk [31, 36] that may be confounders; in our study, we observed an association for RAE that no longer was significant after adjusting for these factors. Finally, adolescent dietary factors could be related to breast cancer but not BBD, due to differences in etiology and/or screening practices.
The current study is limited in that the recall of high school diet ranged from 16 to 38 years later in our study [33]. Although the HS-FFQ has been shown to be moderately reproducible and not strongly correlated with current adult diet, and the modest correlations between the nurses’ and their mothers’ recall of the nurses’ high school dietary intake provided some reassurance [33], the validity of recall many years later has not been established. However, the participants in the NHSII cohort were registered nurses, a population with high levels of health awareness and consciousness, who may be able to recall diet with greater accuracy than the general population. The differences in adolescent dietary recall in our study, particularly in the prospective analysis, should be most likely non-differential and unrelated to BBD diagnosis. Non-differential measurement error in recalled diet would most likely attenuate associations towards the null; thus, we cannot rule out modest true associations. Recently, inverse associations were observed between intakes of vegetable protein, vegetable fat, and carotenoids actually collected during adolescence, and BBD in the prospective Growing Up Today Study [38, 39]. In addition, we previously observed associations of vitamin D, nuts, and fiber with BBD risk, indicating that our questionnaire assessed these foods/nutrients adequately enough to allow for a detectable association [31, 36]. Findings from NHSII may not be directly generalizable to the general population, given that participants are not a random sample of U.S. women. However, we believe epidemiological relations and the underlying biological mechanisms among women in the NHSII are unlikely to differ from women in general. Generalization of the study results to women in other countries is further limited, considering that the dietary pattern of participants in NHSII mainly reflect the North American diet and large difference in dietary patterns across different populations. For instance, an overall dietary pattern rich in fruits and vegetables and carotenoids in China could be quite different from those of western countries [31].
This study has a number of strengths. BBD cases were centrally reviewed by our team of pathologists, which reduced the likelihood of misclassification. We focused on a specific histological subtype of proliferative BBD, a marker of increased breast cancer risk. The results, however, may not be generalizable to other histological subgroups, given the heterogeneity of BBD. The combined analysis includes a large number of proliferative BBD cases. This is the first prospective analysis to evaluate the relation of adolescent dietary intakes of fat, carotenoids, RAE and vitamin E with proliferative BBD risk. Careful control for confounding allowed us to assess the exposures of interest independent of dietary and other BBD risk factors.
In summary, we did not observe associations between high school intakes of fats, carotenoids, or vitamin E with risk of proliferative benign breast disease, even with a large number of cases. Prospectively collected adolescent dietary data and long-term follow-up of BBD are needed to confirm these findings.
Figure 1.
Timeline for completion of high school Food Frequency Questionnaire and benign breast disease selection in NHSII.
Acknowledgements
This work was supported by the Breast Cancer Research Foundation and the National Institutes of Health (grant numbers UM1 CA176726, CA 050385, CA 046475). Caroline Boeke was funded by T32 CA 09001. Graham Colditz was supported by an American Cancer Society Clinical Research Professorship and by the Breast Cancer Research Foundation.
We would like to thank the participants and staff of the Nurses’ Health Study II for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. In addition, this study was approved by the Connecticut Department of Public Health (DPH) Human Investigations Committee. Certain data used in this publication were obtained from the DPH.
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
References
- 1.Armstrong B, Doll R. Environmental factors and cancer incidence and mortality in different countries with special reference to dietary practices. Int J Cancer. 1975;15:617–631. doi: 10.1002/ijc.2910150411. [DOI] [PubMed] [Google Scholar]
- 2.Boyd NF, Martin LJ, Noffel M, Lockwood GA, Trichler DL. A meta-analysis of studies of dietary fat and breast cancer risk. Br J Cancer. 1993;68:627–636. doi: 10.1038/bjc.1993.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howe GR, Hirohata T, Hislop TG, Iscovich JM, Yuan JM, Katsouyanni K, Lubin F, Marubini E, Modan B, Rohan T, et al. Dietary factors and risk of breast cancer: combined analysis of 12 case-control studies. J Natl Cancer Inst. 1990;82:561–569. doi: 10.1093/jnci/82.7.561. [DOI] [PubMed] [Google Scholar]
- 4.Michels KB, Mohllajee AP, Roset-Bahmanyar E, Beehler GP, Moysich KB. Diet and breast cancer: a review of the prospective observational studies. Cancer. 2007;109:2712–2749. doi: 10.1002/cncr.22654. [DOI] [PubMed] [Google Scholar]
- 5.Smith-Warner SA, Spiegelman D, Adami HO, Beeson WL, van den Brandt PA, Folsom AR, Fraser GE, Freudenheim JL, Goldbohm RA, Graham S, et al. Types of dietary fat and breast cancer: a pooled analysis of cohort studies. Int J Cancer. 2001;92:767–774. doi: 10.1002/1097-0215(20010601)92:5<767::aid-ijc1247>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Prentice RL, Caan B, Chlebowski RT, Patterson R, Kuller LH, Ockene JK, Margolis KL, Limacher MC, Manson JE, Parker LM, et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:629–642. doi: 10.1001/jama.295.6.629. [DOI] [PubMed] [Google Scholar]
- 7.Martin LJ, Li Q, Melnichouk O, Greenberg C, Minkin S, Hislop G, Boyd NF. A randomized trial of dietary intervention for breast cancer prevention. Cancer Res. 2011;71:123–133. doi: 10.1158/0008-5472.CAN-10-1436. [DOI] [PubMed] [Google Scholar]
- 8.Frei B. Reactive oxygen species and antioxidant vitamins: mechanisms of action. Am J Med. 1994;97:5S–13S. doi: 10.1016/0002-9343(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 9.McCall MR, Frei B. Can antioxidant vitamins materially reduce oxidative damage in humans? Free Radic Biol Med. 1999;26:1034–1053. doi: 10.1016/s0891-5849(98)00302-5. [DOI] [PubMed] [Google Scholar]
- 10.Blomhoff R. Introduction: overview of vitamin A metabolism and function. In: Blomhoff R, editor. Vitamin A in health and disease. New York: Marcel Dekker, Inc.; 1994. pp. 1–35. [Google Scholar]
- 11.Kelley DS, Bendich A. Essential nutrients and immunologic functions. Am J Clin Nutr. 1996;63:994S–996S. doi: 10.1093/ajcn/63.6.994. [DOI] [PubMed] [Google Scholar]
- 12.Fulan H, Changxing J, Baina WY, Wencui Z, Chunqing L, Fan W, Dandan L, Dianjun S, Tong W, Da P, Yashuang Z. Retinol, vitamins A, C, and E and breast cancer risk: a meta-analysis and meta-regression. Cancer Causes Control. 2011;22:1383–1396. doi: 10.1007/s10552-011-9811-y. [DOI] [PubMed] [Google Scholar]
- 13.Hu F, Wang Yi B, Zhang W, Liang J, Lin C, Li D, Wang F, Pang D, Zhao Y. Carotenoids and breast cancer risk: a meta-analysis and meta-regression. Breast Cancer Res Treat. 2012;131:239–253. doi: 10.1007/s10549-011-1723-8. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Spiegelman D, Baglietto L, Bernstein L, Boggs DA, van den Brandt PA, Buring JE, Gapstur SM, Giles GG, Giovannucci E, et al. Carotenoid intakes and risk of breast cancer defined by estrogen receptor and progesterone receptor status: a pooled analysis of 18 prospective cohort studies. Am J Clin Nutr. 2012;95:713–725. doi: 10.3945/ajcn.111.014415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eliassen AH, Hendrickson SJ, Brinton LA, Buring JE, Campos H, Dai Q, Dorgan JF, Franke AA, Gao YT, Goodman MT, et al. Circulating carotenoids and risk of breast cancer: pooled analysis of eight prospective studies. J Natl Cancer Inst. 2012;104:1905–1916. doi: 10.1093/jnci/djs461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aune D, Chan DS, Vieira AR, Navarro Rosenblatt DA, Vieira R, Greenwood DC, Norat T. Dietary compared with blood concentrations of carotenoids and breast cancer risk: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr. 2012;96:356–373. doi: 10.3945/ajcn.112.034165. [DOI] [PubMed] [Google Scholar]
- 17.Schnitt SJ, Connolly JL. Pathology of benign breast disorders. In: Harris JR, Lippman ME, Morrow M, Osborne CK, editors. Diseases of the breast. 3rd edn. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 77–99. [Google Scholar]
- 18.Rohan TE, Cook MG, Potter JD, McMichael AJ. A case-control study of diet and benign proliferative epithelial disorders of the breast. Cancer Res. 1990;50:3176–3181. [PubMed] [Google Scholar]
- 19.Hislop TG, Band PR, Deschamps M, Ng V, Coldman AJ, Worth AJ, Labo T. Diet and histologic types of benign breast disease defined by subsequent risk of breast cancer. Am J Epidemiol. 1990;131:263–270. doi: 10.1093/oxfordjournals.aje.a115496. [DOI] [PubMed] [Google Scholar]
- 20.Lubin F, Wax Y, Ron E, Black M, Chetrit A, Rosen N, Alfandary E, Modan B. Nutritional factors associated with benign breast disease etiology: a case-control study. Am J Clin Nutr. 1989;50:551–556. doi: 10.1093/ajcn/50.3.551. [DOI] [PubMed] [Google Scholar]
- 21.Webb PM, Byrne C, Schnitt SJ, Connolly JL, Jacobs TW, Baer HJ, Willett WC, Colditz GA. A prospective study of diet and benign breast disease. Cancer Epidemiol Biomarkers Prev. 2004;13:1106–1113. [PubMed] [Google Scholar]
- 22.Rohan TE, Jain M, Miller AB. A case-cohort study of diet and risk of benign proliferative epithelial disorders of the breast (Canada) Cancer Causes Control. 1998;9:19–27. doi: 10.1023/a:1008841118358. [DOI] [PubMed] [Google Scholar]
- 23.Russo J, Tay LK, Russo IH. Differentiation of the mammary gland and susceptibility to carcinogenesis. Breast Cancer Res Treat. 1982;2:5–73. doi: 10.1007/BF01805718. [DOI] [PubMed] [Google Scholar]
- 24.Buell P. Changing incidence of breast cancer in Japanese-American women. J Natl Cancer Inst. 1973;51:1479–1483. doi: 10.1093/jnci/51.5.1479. [DOI] [PubMed] [Google Scholar]
- 25.Colditz GA, Frazier AL. Models of breast cancer show that risk is set by events of early life: prevention efforts must shift focus. Cancer Epidemiol Biomarkers Prev. 1995;4:567–571. [PubMed] [Google Scholar]
- 26.Ziegler RG, Hoover RN, Pike MC, Hildesheim A, Nomura AM, West DW, Wu-Williams AH, Kolonel LN, Horn-Ross PL, Rosenthal JF, Hyer MB. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993;85:1819–1827. doi: 10.1093/jnci/85.22.1819. [DOI] [PubMed] [Google Scholar]
- 27.Frazier AL, Ryan CT, Rockett H, Willett WC, Colditz GA. Adolescent diet and risk of breast cancer. Breast Cancer Res. 2003;5:R59–R64. doi: 10.1186/bcr583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frazier AL, Li L, Cho E, Willett WC, Colditz GA. Adolescent diet and risk of breast cancer. Cancer Causes Control. 2004;15:73–82. doi: 10.1023/B:CACO.0000016617.57120.df. [DOI] [PubMed] [Google Scholar]
- 29.Baer HJ, Schnitt SJ, Connolly JL, Byrne C, Cho E, Willett WC, Colditz GA. Adolescent diet and incidence of proliferative benign breast disease. Cancer Epidemiol Biomarkers Prev. 2003;12:1159–1167. [PubMed] [Google Scholar]
- 30.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6:49–62. doi: 10.1089/jwh.1997.6.49. [DOI] [PubMed] [Google Scholar]
- 31.Su X, Tamimi RM, Collins LC, Baer HJ, Cho E, Sampson L, Willett WC, Schnitt SJ, Connolly JL, Rosner BA, Colditz GA. Intake of fiber and nuts during adolescence and incidence of proliferative benign breast disease. Cancer Causes Control. 2010;21:1033–1046. doi: 10.1007/s10552-010-9532-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trumbo P, Yates AA, Schlicker S, Poos M. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc. 2001;101:294–301. doi: 10.1016/S0002-8223(01)00078-5. [DOI] [PubMed] [Google Scholar]
- 33.Maruti SS, Feskanich D, Colditz GA, Frazier AL, Sampson LA, Michels KB, Hunter DJ, Spiegelman D, Willett WC. Adult recall of adolescent diet: reproducibility and comparison with maternal reporting. Am J Epidemiol. 2005;161:89–97. doi: 10.1093/aje/kwi019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312:146–151. doi: 10.1056/NEJM198501173120303. [DOI] [PubMed] [Google Scholar]
- 35.Willett W, Stampfer M. Implications of total energy intake for epidemiologic analyses. In: Willett W, editor. Nutritional Epidemiology. 2nd edn. New York: Oxford University Press; 1998. pp. 273–301. [Google Scholar]
- 36.Su X, Colditz GA, Collins LC, Baer HJ, Sampson LA, Willett WC, Berkey CS, Schnitt SJ, Connolly JL, Rosner BA, Tamimi RM. Adolescent intakes of vitamin D and calcium and incidence of proliferative benign breast disease. Breast Cancer Res Treat. 2012;134:783–791. doi: 10.1007/s10549-012-2091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Therneau TM. Extending the Cox Model. In: Lin DY, Fleming TR, editors. Proceedings of the First Seattle Symposium in Biostatistics: Survival Analysis. New York: Springer Verlag; 1997. pp. 51–84. [Google Scholar]
- 38.Boeke CE, Tamimi RM, Berkey CS, Colditz GA, Eliassen AH, Malspeis S, Willett WC, Frazier AL. Adolescent carotenoid intake and benign breast disease. Pediatrics. 2014;133:e1292–e1298. doi: 10.1542/peds.2013-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berkey CS, Willett WC, Tamimi RM, Rosner B, Frazier AL, Colditz GA. Vegetable protein and vegetable fat intakes in pre-adolescent and adolescent girls, and risk for benign breast disease in young women. Breast Cancer Res Treat. 2013;141:299–306. doi: 10.1007/s10549-013-2686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagel G, Linseisen J, van Gils CH, Peeters PH, Boutron-Ruault MC, Clavel-Chapelon F, Romieu I, Tjønneland A, Olsen A, Roswall N. Dietary beta-carotene, vitamin C and E intake and breast cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) Breast Cancer Res Treat. 2010;119:753–765. doi: 10.1007/s10549-009-0444-8. [DOI] [PubMed] [Google Scholar]