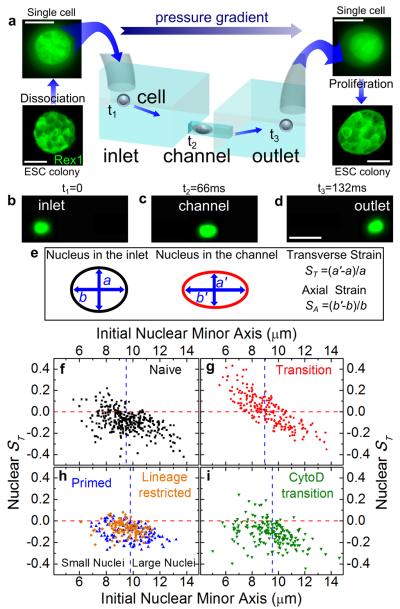
Fig. 2. Optofluidic assay.
(a) ESCs are detached from culture plate, resuspended as single cells and loaded in the inlet of the microfluidic chip. The applied pressure gradient powers the transport of single cells from the inlet to the outlet. The inlet and outlet reservoirs are connected via channels smaller than the average cell size; therefore, cells must squeeze through the channels. Single cells collected from the outlet form colonies of fully functioning ESCs. Scale bars: 10 and 50 μm for single cells and colonies respectively. (b)-(d) Three consecutive epi-fluorescence snapshots of a Syto13-labelled cell translocating the channel from the inlet to the outlet under an applied pressure of 10 mbar. The detected fluorescence signal allows the simultaneous measurement of both cell and nuclear deformation. Scale bars: 25 μm. (e) Schematic of nuclear shape whilst the cell is the inlet (left) and in the channel (right). We quantify nuclear deformation by using the transverse, ST, and axial strains, SA, where a (b) and a’ (b’) are the nuclear transverse (axial) extents in the inlet and in the channel, respectively. (f)-(i) Scatter plots correlating the transverse nuclear strain ST in the channel to the initial nuclear minor axis for N-ESCs (squares), T-ESCs (circles), P-cells (upward triangles), lineage restricted HL60 cells (diamonds) and T-ESCs treated with Cytochalasin D (downward triangles). M= 3, 3, 2, 1 and 2, respectively. A negative (positive) strain denotes a decrease (increase) of the nuclear transverse extent while the cell squeezes through the channel. The horizontal lines denotes a nuclear ST of zero, while the vertical line is the population median for each data set used to separate the population of each class of cells in small and large nuclei categories. Small T-ESC nuclei show a markedly different behavior from all other investigated cell types: their ST increases above 0, indicative of auxeticity.