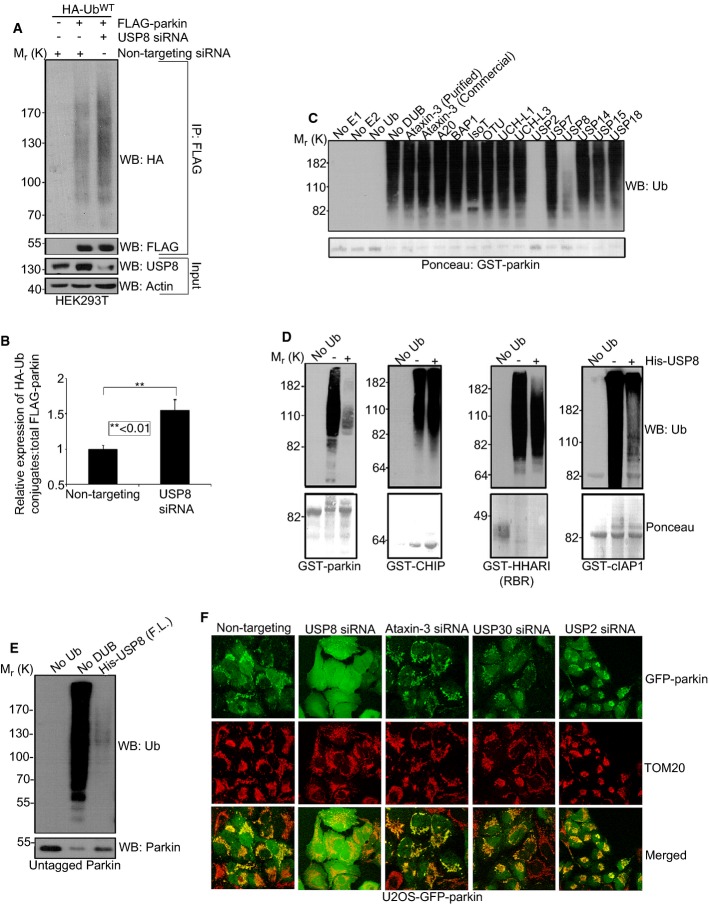
Figure 3. USP8 deubiquitinates parkin.
A, B USP8 knockdown causes Ub conjugates to accumulate on FLAG-parkin in HEK293T cells. HEK293T cells were co-transfected with FLAG-parkin (0.5 μg), HA-UbWT (0.5 μg) and non-targeting or USP8 siRNA (5 nM). Lysates were immunoprecipitated with FLAG resin and analyzed by immunoblotting for HA and FLAG. Input lysates (5% of total input) were analyzed by immunoblotting for USP8 and actin. The optical densities of the Ub-parkin conjugates were quantified using NIH ImageJ, and the data represent the mean ± SEM for three independent experiments. For statistical analysis, a two-way ANOVA with Tukey post-test was performed, **P < 0.01.
C The activity of USP8 toward parkin relative to other DUBs. GST-parkin bound to glutathione beads was left to ubiquitinate for 2 h alone at 37°C. After 2 h, the beads were washed to remove reaction components and ubiquitinated GST-parkin was then incubated in the presence or absence of the indicated DUB for 1 h at 37°C. Reactions were immunoblotted for Ub and Ponceau stained for GST-parkin.
D USP8 preferentially hydrolyzes preassembled parkin Ub conjugates. For these reactions, GST-parkin, GST-CHIP, GST-HHARI (RBR domain), or GST-cIAP1 bound to glutathione beads were left to ubiquitinate for 2 h alone at 37°C. After 2 h, the beads were washed to remove reaction components. The ubiquitinated E3 was then incubated in the presence or absence of the full-length His-USP8 for 1 h at 37°C. Reactions were analyzed by Ponceau S staining and immunoblotting for Ub.
E USP8 can hydrolyze preassembled Ub conjugates on untagged parkin. For these reactions, untagged parkin was left to ubiquitinate for 2 h alone at 37°C with UbcH7 as the E2. After 2 h, apyrase was added for 20 min to terminate the reaction. Ubiquitinated parkin was now incubated for 1 h at 37°C in the presence or absence of His-tagged full-length USP8. Reactions were immunoblotted for Ub and parkin.
F Knockdown of other DUBs compared to USP8 had no effect on the recruitment of parkin onto mitochondria following CCCP treatment. U2OS-GFP-parkin cells were transfected with either non-targeting, USP8, ataxin-3, USP30, or USP2 siRNA (10 nM) for 60 h. Cells treated with CCCP for 1 h were fixed, and images were acquired after staining for the mitochondrial protein, TOM20 (see also Supplementary Fig S6).
Source data are available online for this figure.