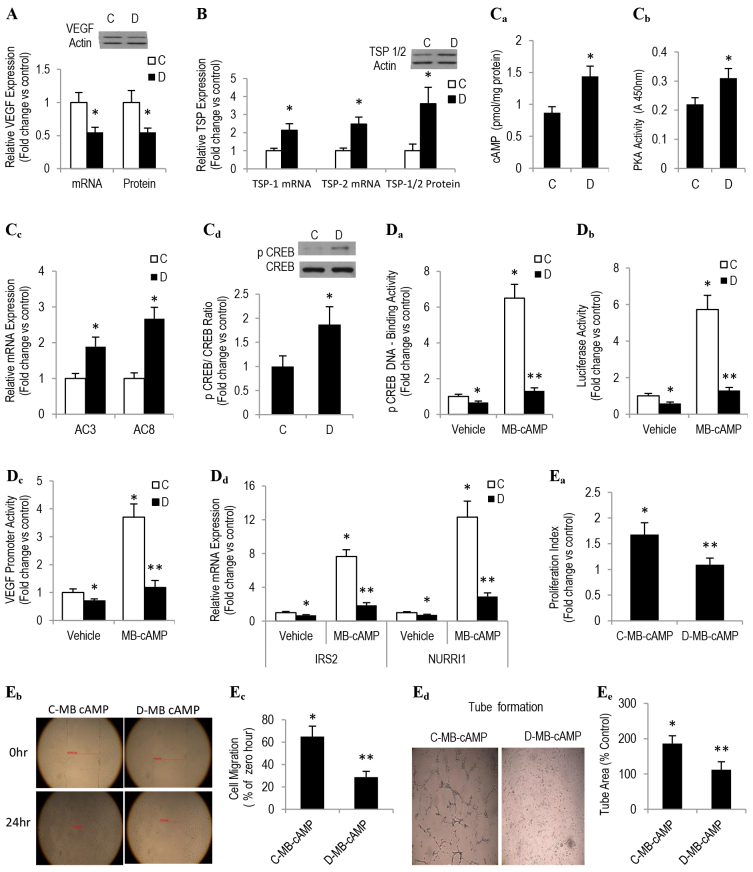
Fig. 3.
Diabetes antagonizes angiogenesis by altering PKA-CREB-VEGF-dependent signaling. Endothelial cells isolated from sponge implants (SIECs) from control and diabetic mice were used to assess angiogenic capacity and the cAMP-PKA-dependent pathway. qRT-PCR- and western-blotting-based techniques were used in the determination of mRNA (lower graph) and protein levels (upper blot), respectively, of (A) VEGF and (B) TSP1 and TSP2. (Ca) cAMP level was measured using ELISA, (Cb) PKA activity was measured using enzyme activity and (Cc) AC3 and AC8 mRNA expression was measured qRT-PCR. (Cd) Similarly, total cellular contents of p-CREB were assessed by western blot analysis. (Da) pCREB-DNA binding activity in nuclear extracts was assessed by a TransAM-ELISA-based assay. (Db,c) SIECs were transiently transfected with the luciferase reporter plasmid containing the (Db) CRE or (Dc) VEGF promoter and then treated with 100 μM MB-cAMP for 6 hours (CRE) or 12 hours (VEGF), and the relative luciferase activity in cell extracts was measured with a luminometer. (Dd) The mRNA expression of a number of CREB target genes containing a conserved CRE was determined by using real-time PCR analyses. Angiogenic capacity was evaluated in terms of (Ea) cell proliferation (e.g. 24-hour starved cells were treated with MB-cAMP for 24 hours in the presence of 10 μM BrdU followed by fixation and assaying the rate of BrdU incorporation into DNA; (Eb,c) cell migration (e.g. scratching 24-hour starved cells with a pipette tip followed by measuring the percentage of the wound covered by cells under a light microscope; Eb shows example images, Ec shows the quantitation) and (Ed,e) tube formation (e.g. serum-starved cells were seeded on growth-factor-reduced Matrigel. Ed shows example photographs that were taken after 24 hours, Ee shows the quantitation). ‘C’, control; ‘D’, diabetic. Results are expressed as means±s.e.m. from three independent experiments. *Significantly different from corresponding vehicle-treated control values at P≤0.05. **Significantly different from corresponding MB-cAMP-treated control values at P≤0.05.