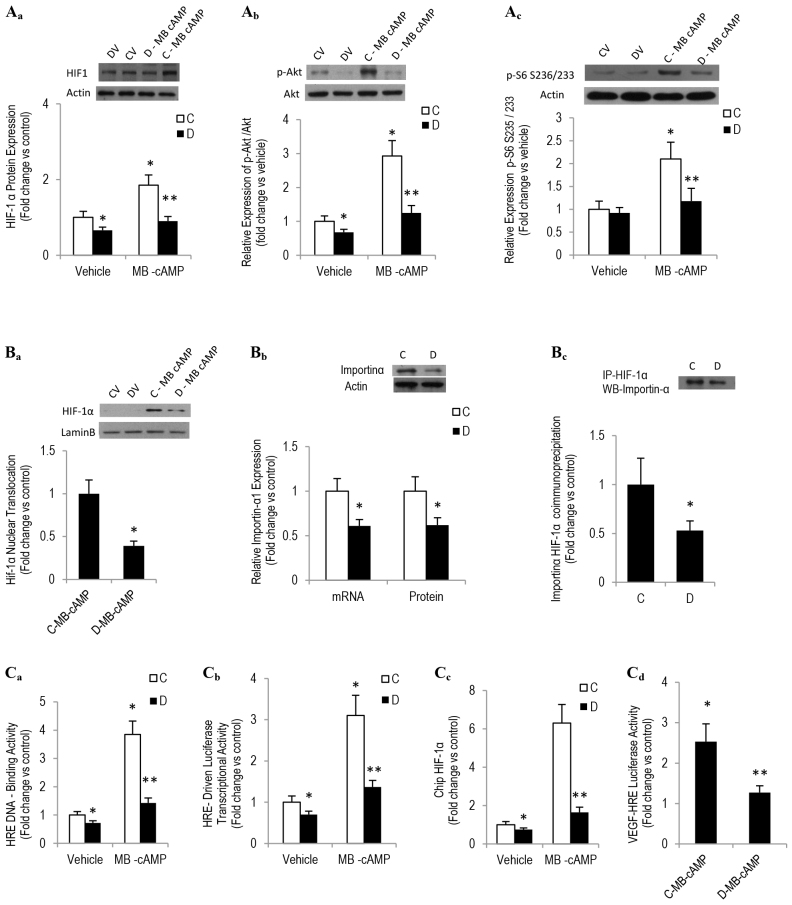
Fig. 4.
Altered HIF-1α dynamics contribute to reduced VEGF expression in diabetic SIECs. (Aa–c) Total cell contents of HIF-1α, pAkt (at residue S473) and pS6 (at residues S235/236) were assessed in SIECs that had been exposed to MB-cAMP for 16 hours. Example blots are shown at the top, quantitation of the blots is shown in the graphs below. (Ba) HIF-1α nuclear localization in SIECs treated with MB-cAMP for 2 hours was determined using a cell fractionation kit (Active Motif). A representative blot is shown, and quantitation of the blot is shown underneath. (Bb) Similarly, importin α mRNA and protein expression in SIECS was assessed using qRT-PCR (graph) and western blotting (blot). (Bc) The binding affinity of HIF-1α for importin α was evaluated by using co-immunoprecipitation followed by western blotting. The graph shows the quantitation of the blot. (Ca) The transcription factor ELISA assay revealed that MB-cAMP (100 μM) elicited the specific binding of SIEC nuclear extracts to the HRE consensus in a time-dependent manner, which was inhibited by wild-type but not mutated HRE oligonucleotide. (Cb) SIECs were transfected with a HRE-driven luciferase reporter construct and Renilla luciferase control plasmid for 24 hours. After transfection, cells were exposed to MB-cAMP (100 μM) for 12 hours and the intensity of luciferase reactions normalized to their Renilla luciferase control activity was measured using a dual luciferase assay kit. (Cc) A ChIP assay showed an interaction of HIF-1α with the HRE-containing promoter region of VEGF in SIECs that had been treated with MB-cAMP (100 μM) for 4 hours. (Cd) SIECs were transiently transfected with a plasmid expressing the reporter gene luciferase under the control of a fragment of the VEGF prompter containing the HRE, and then the intensity of the luciferase activity was measured 24 hours following MB-cAMP exposure. ‘C’, control; ‘D’, diabetic. Results are expressed as means±s.e.m. for three independent experiments. *Significantly different from corresponding control values at P≤0.05. **Significantly different from corresponding MB-cAMP-treated control values at P≤0.05.