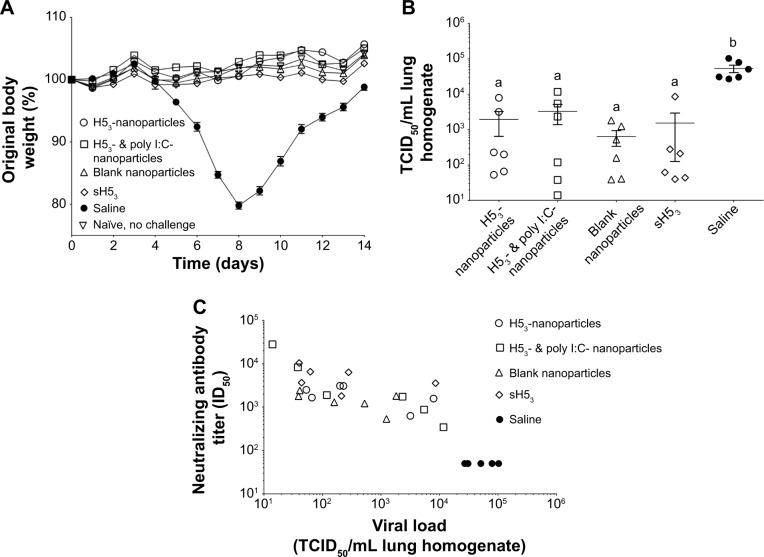
Figure 5.
Mice were protected upon challenge with low pathogenic VNH5N1-PR8/CDC-RG.
Notes: (A) Mice were challenged with a reverse genetics-derived influenza virus, VNH5N1-PR8/CDC-RG, containing the HA and NA genes of A/Vietnam/1203/04 (H5N1) virus in the genetic background of the high-growth master strain A/Puerto Rico/8/34 (H1N1). Body weight was observed for 2 weeks postinfection. All vaccinated mice maintained or gained weight similar to healthy naïve, no challenge mice. (B) Viral load in lung homogenates collected at 3 days post-challenge, (C) which correlated with the pre-challenge neutralizing antibody titers. Different letters indicate statistical significant among treatments.
Abbreviations: H53, H5 hemagglutinin trimer; HA, hemagglutinin; ID50, sera dilution that inhibits 50% of pseudovirus infectivity; NA, neuraminidase; sH53, soluble H5 hemagglutinin trimer; TCID50, tissue culture infectious dose 50%.