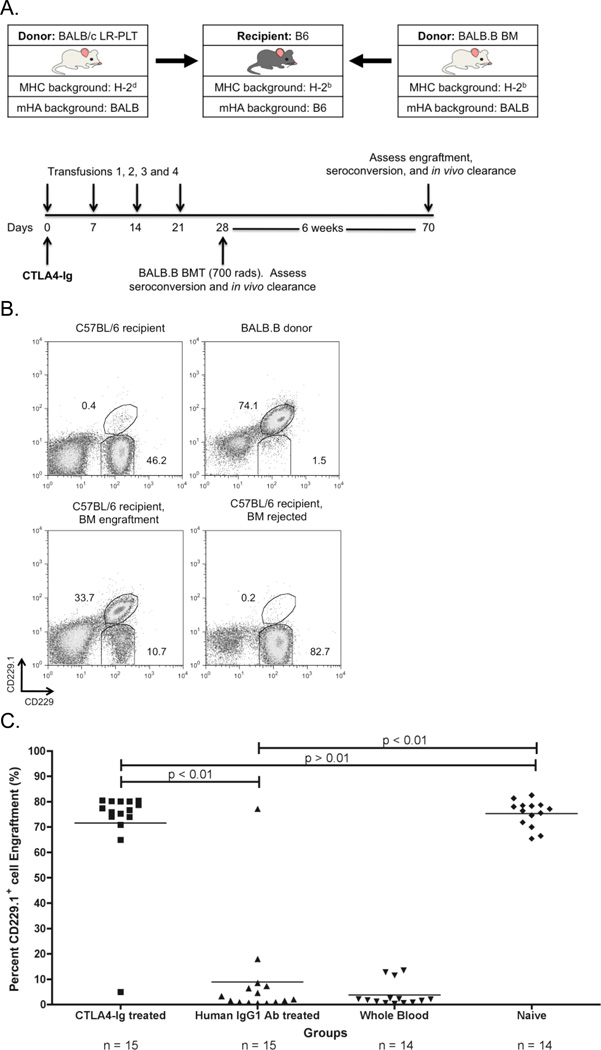
Figure 3. CTLA4-Ig LR-PLT transfusion induced BMT rejection.
(A) Experimental model testing the ability of CTLA4-Ig to prevent platelet induced rejection of an MHC-matched BMT. BALB/c platelet donors were MHC- and mHA-mismatched, whereas BALB.B bone marrow donors were MHC-matched but mHA-mismatched with respect to the C57BL/6 recipients. Recipients received a single 500 µg dose of CTLA4-Ig or human IgG1 i.p. two hours prior to the first transfusion. Recipients received four LR-PLT transfusions, a week apart. After the fourth transfusion, recipients received a BALB.B BMT under reduced intensity conditions. in vivo survival of BALB.B targets was performed after BMT (see Figure 4). (B) The CD229+ congenic markers. Representative dot plots illustrate the ability to detect C57BL/6 recipient CD229+ (top left panel) and BALB.B donor CD229.1+ (right panel) cells by flow cytometry. Engraftment is demonstrated as the presence of a double positive CD229.1+ CD229+ population (bottom left panel) and rejection as the absence of this double positive population (bottom right panel). Chimerism is indicated by the presence of both the double positive CD229.1+ CD229+ and the single positive CD229+ populations. (C) BALB.B BMT engraftment results. Engraftment is represented as percent CD229.1+ cells in the peripheral blood; the horizontal lines denote the mean of each group. Rejection was measured as having a percent CD229.1+ cells engraftment two standard deviations above the mean of the positive control group known to reject, recipients treated with isotype control antibody human IgG1. Statistics were generated using column statistics and a one-way ANOVA with Dunnett’s post-test. The combined data from three independent experiments are shown.