Abstract
Backgrounds and Aims Leaf functional traits have been used as a basis to categoize plants across a range of resource-use specialization, from those that conserve available resources to those that exploit them. However, the extent to which the leaf functional traits used to define the resource-use strategies are related to root traits and are good indicators of the ability of the roots to take up nitrogen (N) are poorly known. This is an important question because interspecific differences in N uptake have been proposed as one mechanism by which species’ coexistence may be determined. This study therefore investigated the relationships between functional traits and N uptake ability for grass species across a range of conservative to exploitative resource-use strategies.
Methods Root uptake of and , and leaf and root functional traits were measured for eight grass species sampled at three grassland sites across Europe, in France, Austria and the UK. Species were grown in hydroponics to determine functional traits and kinetic uptake parameters (Imax and Km) under standardized conditions.
Key Results Species with high specific leaf area (SLA) and shoot N content, and low leaf and root dry matter content (LDMC and RDMC, respectively), which are traits associated with the exploitative syndrome, had higher uptake and affinity for both N forms. No trade-off was observed in uptake between the two forms of N, and all species expressed a higher preference for .
Conclusions The results support the use of leaf traits, and especially SLA and LDMC, as indicators of the N uptake ability across a broad range of grass species. The difficulties associated with assessing root properties are also highlighted, as root traits were only weakly correlated with leaf traits, and only RDMC and, to a lesser extent, root N content were related to leaf traits.
Keywords: Ammonium, nitrate, plant functional traits, leaf traits, root traits, root nitrogen uptake, 15N labelling, uptake rate, affinity, grasses, Poaceae, grassland ecology, resource-use strategy
INTRODUCTION
Plant resource-use strategies have received substantial attention over the last two decades as a means to better understand and predict the dynamics and functioning of ecosystems, particularly in the context of global change (Reich, 2014). It has been proposed that plant strategy theories (Grime, 1977; Tilman, 1990; Westoby et al., 2002) can be underpinned by quantitative plant functional traits. In particular, some leaf traits, such as specific leaf area (SLA) or leaf dry matter content (LDMC), have been considered as indicators of a plant’s capacity to acquire, use and recycle resources (Weiher et al., 1999; Wright et al., 2001). These traits are closely related to species photosynthetic efficiency and relative growth rate (Reich et al., 1999; Garnier et al., 2001; Wright et al., 2004), as well as leaf life span (Reich et al., 1999; Ryser and Urbas, 2000). The ‘leaf economics spectrum’ (Wright et al., 2004) captures such relationships by highlighting the link between SLA or leaf nitrogen concentration (LNC), and a spectrum running from an exploitative syndrome, with rapid turnover of nutrients in leaves resulting in fast growth, to a conservation syndrome associated with the conservation of nutrients in well-protected tissues and a resultant slow growth (Chapin, 1980; Poorter and De Jong, 1999; Wright et al., 2004).
The existence of such a trade-off between acquisition and conservation of resources has also been suggested to occur for root traits (Roumet et al., 2006; Fort et al., 2013), and while relationships between root traits and functional processes linked to plant strategies are not as well documented as for leaves, several recent studies demonstrate the value of root traits as indicators of functions. Birouste et al. (2012), for example, found a relationship between root chemical composition and root potential decomposition rate for Mediterranean herbaceous species, and Makita et al. (2012), studying the root systems of forest trees, identified relationships between root tissue density and specific root length (SRL), and coarse root respiration. Although specific environmental constraints may differentially affect above-ground and below-ground organs (Craine et al., 2005; Tjoelker et al., 2005; Roumet et al., 2006; Chen et al., 2013), integrated above- and below-ground syndromes can be linked, and collectively provide an explanation for resource-use strategies observed for the whole plant (Freschet et al., 2010; Liu et al., 2010; Fortunel et al., 2012; Reich et al., 2014).
Relationships between root N uptake and plant resource-use strategies as described by leaf traits have not yet been clearly established. Exploitative species are assumed to have high abilities to take up nutrients, as evidenced by the positive correlation between growth rate and N concentration in plants (Poorter et al., 1990; Garnier, 1991). Some studies have strengthened this assumption by showing a link between the N absorption rate on the one hand, and SLA and/or leaf N concentration on the other (Osone et al., 2008; James et al., 2009; Leffler et al., 2013). Root N uptake may depend on two components: root system morphology, which determines access to resources within soils, and physiological traits driving influx capacities. Root metabolic activity and nutrient uptake have indeed been related to root structural and chemical traits such as SRL or N concentration (Comas and Eissenstat, 2004; Tjoelker et al., 2005; Bahn et al., 2006; Maire et al., 2009). It is now well established that the high affinity transport system transport (HATS) contributes mainly to the N uptake at low to moderate concentrations of external N and saturates at 0·2–0·5 mm (Kronzucker et al., 2000; Min et al., 2000), which makes it the more probable system used by plants growing in natural and semi-natural ecosystems limited by N (Bassirirad, 2000; Maire et al., 2009). Thus, the uptake of and can be described by two kinetic parameters, the maximum uptake rate (Vmax) and the affinity constant or Michaelis constant (Km), to determine the ability and the efficiency of roots in absorbing and . The Michaelis constant is commonly interpreted as the substrate ( or ) concentration required to achieve 50 % of Vmax and/or as the measure of HATS affinity for its substrate (low Km values being equivalent to higher affinity). While some studies provide indirect support for the idea that slow-growing plants occurring in nutrient-poor environments have a higher affinity of transport systems for N (lower Km) (Garnier et al., 1989; Muller and Garnier, 1990), others have not found any evidence to support such a relationship (Freijsen and Otten, 1984; Bloom, 1985; Oscarson et al., 1989). In addition, due to a lack of comparative studies characterizing Km under standardized conditions for a range of species, the relationship between Km for N and species resource-use strategies (Lambers and Poorter, 1992) is still poorly understood. Such studies would also be needed to determine whether higher affinity (low Km values), which allows species to exploit N resources at lower soil concentrations, is a characteristic of slow-growing species or a characteristic of fast-growing species.
The overall goal of our study was to test for relationships between N uptake ability and functional traits of a broad range of grass species representing contrasting resource-use strategies. To do this, we grew plants taken from three grassland sites across Europe under standardized conditions, and measured their leaf and root functional traits, and estimated uptake parameters (maximum uptake rate, Imax; and Michaelis–Menten affinity constant, Km) for the main inorganic N forms available for plant nutrition, ammonium () and nitrate (). Specifically, we addressed the following questions. (1) What are the relationships between root functional traits and the resource-use strategy of species as described by leaf traits? (2) Are exploitative species more efficient in taking up and (higher Imax) compared with conservative species? (3) Do conservative species, which generally grow in N-poor environments, have higher affinity (lower Km) for both and than exploitative species? We hypothesized that exploitative species are more efficient in acquiring N when its availability is high (high uptake rate), and conservative species are more efficient in acquiring N when its availability is low (low uptake rate but higher affinity).
MATERIALS AND METHODS
Plant species and cultures
Eight grass species were chosen to represent common species at three European grassland sites encompassing a large range of functional diversity (see Grigulis et al., 2013 for detailed site descriptions). Briefly, the three sites represented different levels of overall grassland management intensity, and were dominated by different species ranging from conservative to exploitative strategies. Briza media, Bromus erectus, Dactylis glomerata, Festuca paniculata and Sesleria caerulea were sampled at the French site located in the French Alps, near the Lautaret Pass (45 °2′5·1′′N, 6 °22′43·5′′E, elevation 1700–2000 m). Dactylis glomerata and Nardus stricta were sampled at the Austrian site located in the Stubai Alps (47 °7′46·10′′N, 11 °18′21·60′′E, elevation: 1800–2000 m). Anthoxanthum odoratum, Dactylis glomerata and Lolium perenne were sampled at the UK site located in the Yorkshire Dales (UK) (54 °18′31·8′′N, 2 °4′53·8′′, elevation: 200 m). Dactylis glomerata was collected across all sites as a control to ensure that observed differences between species would be linked to differences in plant strategies and not to the origins of plants.
For each species, a few individuals (3–5) were sampled in the field and vegetatively multiplied on floating perlite at the University of Caen in a greenhouse (16 h day 20 °C/8 h night 16 °C) with additional light provided by sodium lamps (400 W Philips SON T-PIA Agro, providing 400 μmol m−2 s−1 photosynthetically active radiation). Plants were supplied with a nutritive solution renewed every week that contained 1 mm NH4NO3, 0·18 mm CaCO3, 0·4 mm KH2PO4, 0·15 mm K2HPO4, 3 mm CaCl2, 0·2 mm EDTA, 2NaFe·H2O, 14 μm H3BO3, 5 μm MnSO4·H2O, 3 μm ZnSO4·7H2O, 0·7 μm CuSO4·5H2O, 0·7 μm (NH4)6Mo7O24 and 0·1 μm CaCl2. During the multiplication step, we regularly cut (5 cm) the aerial and root parts in order to favour the production of new tissues. This multiplication step ran for 2–3 months depending on the growth of species, and was designed to standardize the growing conditions of plants and species coming from different sites as well as to provide enough replicates to estimate N uptake (approx. 100 tillers per species). Before the labelling experiment, several tillers used as replicates were grown in hydroponic culture with the same solution renewed every 3 d to prevent any nutrient limitation, and without cutting. This last period of growth ran until individuals reached the stage of 3–4 tillers (usually between 2 and 3 weeks) in order to ensure a similar phenological status for all species. We assumed that the larger number of replicate tillers randomly used for the different analyses represented the genetic variation of the mother plants sampled in the field.
Nitrogen labelling and functional traits
15N labelling was used to determine kinetic parameters of and uptake by the different plants studied. Following Glass (2000), kinetic parameter measurements were applied to determine incoming 15N influx (rather than net uptake, which is the resultant of influx and efflux). For this reason, the duration of 15N labelling did not exceed 5 min. The plant roots were washed twice for 1 min with a solution of calcium sulphate (CaSO4; 1 mm), then transferred over 5 min to a nutritive solution containing 15NO3 (K15NO3; 15N excess = 99 %) or 15NH4 [(15NH4)2SO4; 15N excess = 99 %], then washed again twice for 1 min with a 1 mm CaSO4 solution maintained at 4 °C to stop any energetic processes. We labelled the whole root plant system across a range of concentrations (<1 mm) relevant to estimate HATS activity and kinetic N uptake parameters of plants. For each species and N form, kinetic parameters of and uptake were determined from three sets of measurement. Each set of measurements corresponds to one N kinetic uptake, obtained by cultivating several replicates obtained after the multiplication phase for 5 min at the different labelled N concentrations along an increasing gradient(20–30–40–50–75–100–150–250–500–750 μm), providing at least 30 ‘replicates’ along the gradient to estimate the N uptake kinetics for each N form (see below). During the labelling, roots were attached to the shoot; therefore, our measurements represent the whole-plant N uptake. At the end of the labelling, shoots and roots of all replicates were weighed and analysed separately.
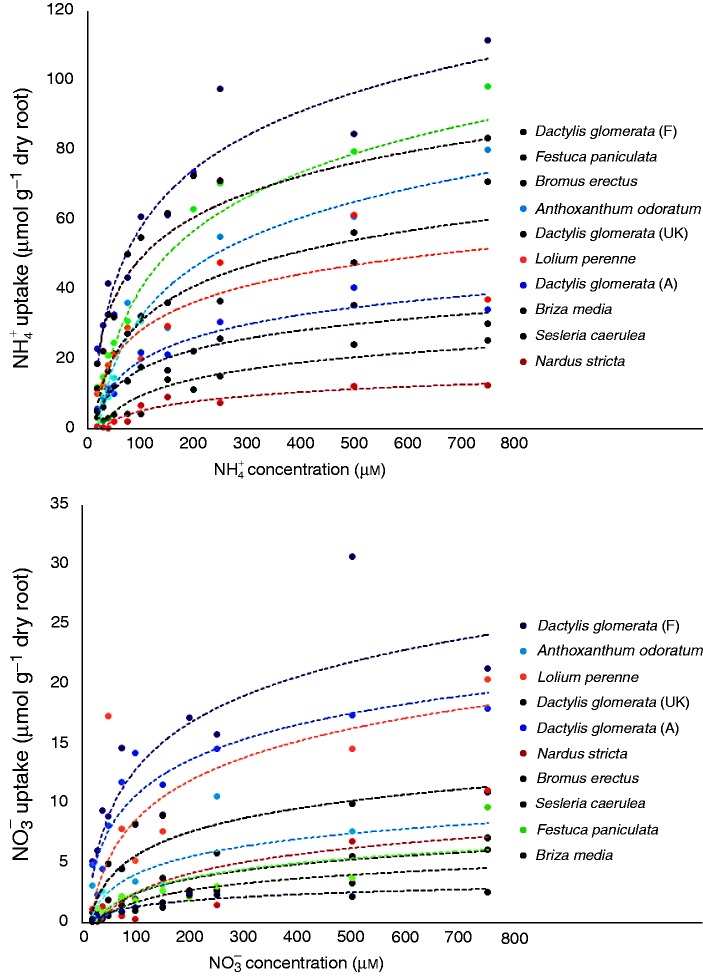
The total N amount and 15N excess were determined by analysing samples after oven-drying at 60 °C during 72 h and grinding to a fine powder, with a continuous flow isotope mass spectrometer (Isoprime, GV Instruments, Manchester, UK) linked to a C/N/S analyser (EA3000, Euro Vector, Milan, Italy). The total N (Ntot) content of a given replicate ‘i’ was calculated as: Ntoti = ( %Ni × DMi)/100. The natural 15N abundance (0·3663 ± 0·0004 %) of atmospheric N2 was used as a reference for 15N analysis. Nitrogen derived from current N uptake (Q15N) in a given replicate was calculated as: Q15N = (Ntoti × Ei)/Es, where Ei ( %) is the atom 15N excess and Es is the nutrient solution atom 15N excess. Then, and influx was expressed as μmol h–1 g–1 dry root or μmol h–1 g–1 dry root, respectively (Fig. 1).
Fig. 1.
Nitrogen uptake rates of (top) and (bottom) at the different substrate concentrations. Species names in the key are ordered following the position of the regression line at the end of the N concentration axis to simplify the reading of the figure. However, the order was not necessarily the same as the ranking based on Vmax values presented in Fig. 3, which were calculated using all the points and not only the mean values per concentration presented here.
We measured leaf and root functional traits using standardized protocols (Cornelissen et al., 2003) for 6–10 replicates of each species (see Table 1) similar to those used for N uptake estimation. Because plants were grown under optimal conditions in hydropony, we did not rehydrate leaves before measurement, but kept leaves in ice until measurement, <2 h after harvest. Leaf area (LiCor 3100), fresh mass and dry mass (after drying at 60 °C during 48 h) of the last mature leaf were measured to assess SLA and LDMC. Fresh mass and dry mass of roots as well as root length (Winrhizo® software) were measured to assess root dry matter content (RDMC) and SRL. The allocation of biomass to shoots or roots was evaluated by the ratio of their dry masses, i.e. the shoot:root ratio (SRR).
Table 1.
Species effect on the different traits
| Species | SLA (mm2 mg–1) | LDMC (mg g–1) | Nshoot (%) | SRL (m g–1) | RDMC (mg g–1) | Nroot (%) | SRR |
|---|---|---|---|---|---|---|---|
| Anthoxanthum odoratum (10) | 21·93ab (1·91) | 297·49bc (23·11) | 4·19a (0·17) | 79·48ab (9·71) | 77·93d (2·99) | 4·40ab (0·16) | 10·44ab (0·96) |
| Briza media (6) | 11·05c (1·09) | 326·80ab (22·18) | 3·37bc (0·16) | 116·54ab (12·31) | 118·47abc (2·65) | 3·55bc (0·15) | 2·88c (0·32) |
| Bromus erectus (6) | 14·37c (1·36) | 262·10 b (15·45) | 4·27 a (0·30) | 104·42ab (13·91) | 133·74ab (7·23) | 4·74a (0·49) | 2·62c (0·21) |
| Dactylis glomerata (A) (10) | 20·60b (1·65) | 227·17c (7·52) | 4·09a (0·13) | 47·98b (7·44) | 131·47ab (12·31) | 2·86c (0·17) | 3·38c (0·27) |
| Dactylis glomerata (F) (6) | 26·88a (1·69) | 204c (12·62) | 3·80ab (0·2) | 105·46ab (18·26) | 119·19ab (9·50) | 2·93c (0·17) | 4·00bc (0·35) |
| Dactylis glomerata (UK) (10) | 24·74ab (1·09) | 215·89c (8·36) | 4·14a (0·08) | 56·49b (4·81) | 71·56d (3·49) | 2·98c (0·15) | 3·63bc (0·27) |
| Festuca paniculata (6) | 8·81cd (1·35) | 321·25b (32·65) | 2·82cd (0·15) | 150·26a (54·55) | 159·63a (21·42) | 3·23c (0·22) | 12·14a (2·36) |
| Lolium perenne (10) | 15·41c (0·64) | 275·10bc (10·68) | 4·20a (0·05) | 98·23a (14·32) | 90·50cd (2·38) | 4·23ab (0·08) | 4·26bc (0·16) |
| Nardus stricta (10) | 4·18d (0·17) | 435·97a (35·54) | 2·53d (0·14) | 41·88b (5·57) | 151·82ab (20·50) | 1·89d (0·21) | 1·64c (0·16) |
| Sesleria caerulea (6) | 9·55cd (1·22) | 358·40ab (18·65) | 3·23bc (0·11) | 62·29b (21·21) | 115·04bc (12·67) | 2·74cd (0·23) | 7·06b (1·49) |
| F-ratio | 36·88 | 14·01 | 22·25 | 4·48 | 15·95 | 21·99 | 22·87 |
Mean and standard error in parentheses are given for functional traits for each species.
The number of replicates is indicated for each species in parentheses.
The F-ratio (P-value <0·001 for all traits) after an ANOVA testing the differences between species for each trait ia indicated (n = 80).
See Fig. 2 and the text for a full description of the trait acronyms.
Kinetic uptake parameter estimation
Kinetic uptake parameters (Imax, maximum influx; Km, Michaelis constant) for HATS were determined to compare the efficiency of species to take up and from the solution. Following Engels et al. (2000), Hanes’s relationship (Michaelis–Menten transformed equation) was used to fit the dependency of the influx rate on the substrate concentration (C; μm) allowing the direct calculation of the maximal influx rate of or from the slope of the linear curve: 1/influx = Km/Imax × C + 1/Imax.
The specific influx capacity was calculated as the maximal influx rate per unit of root dry mass. Substrate affinity (Km) of the HATS was calculated from the intercept divided by the slope of the linear curve. Km translates the efficiency to take up N inorganic compounds at low concentrations: a low Km value characterizes a high affinity of HATS for or .
Data analysis
Relationships between functional traits were assessed using Pearson correlation coefficients. A principal component analysis (PCA) was used to explore the distribution of species relative to their functional traits and uptake parameters for and . Species differences for uptake parameters were tested using a one-way analysis of variance (ANOVA), followed by a Tukey post-hoc test. All statistical analyses were performed with the statistical software R 2·14 (R Development Core Team, 2012), and the PCA analysis was carried out with the ade4 package (Chessel et al., 2004).
RESULTS
Leaf and root trait syndromes
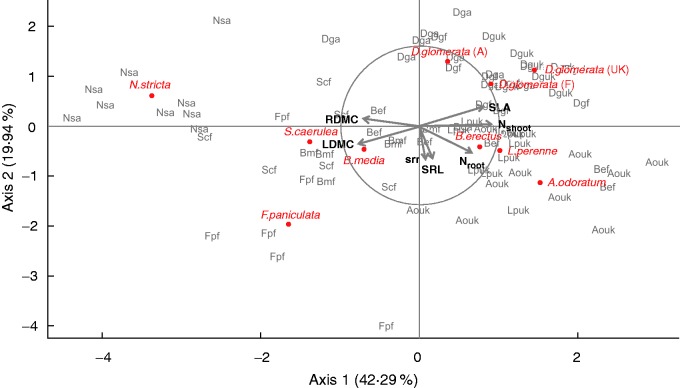
The studied species encompassed a large spectrum of variability for functional traits, with SLA ranging from 4 to 27 mm2 g–1, LDMC from 204 to 436 mg g–1 and Nshoot from 2·5 to 4·3 mg g–1 (Table 1). Variations for LDMC, SLA and Nshoot in our experiment represented 35, 28 and 27 %, respectively, of the variation reported for a wider range of species and life forms in Cornelissen et al. (2003). In the PCA (Fig. 2), species spanned mainly along the first axis (42·29 % of total inertia) from a conservative syndrome with high values of LDMC and RDMC associated with low values of SLA and Nshoot for species such as N. stricta, S. caerulea and F. paniculata, to an exploitative syndrome with high values of SLA and Nshoot associated with kinetic low values of LDMC and RDMC for species such as D. glomerata or A. odoratum. The second axis (19·94 % of total inertia) was related to variations in SRR and SRL. Consequently, SRR and SRL were orthogonal to the first axis and thus independent of species strategies. Both SRR and SRL are indeed not correlated with the main contributing traits to the first axis (Nshoot, SLA, LDMC and RDMC). Among traits, the strongest correlation was the negative one between SLA and LDMC (r = –0·76, P < 0·001), two main contributors to the leaf economics spectrum. Those leaf traits were correlated with Nshoot (with SLA, r = 0·60, P < 0·001, and with LDMC, r = –0·58, P < 0·001). Among root traits, only RDMC was well correlated to the leaf economics spectrum (with SLA, r = –0·46, P < 0·001; with LDMC, r = 0·30, P < 0·01; and with Nshoot, r = –0·57, P < 0·001). Of SRL and Nroot, which were chosen to characterize economic aspects of the root system, the second was the more relevant trait when studying plant strategies, because of high correlation with SRL (r = 0·30, P < 0·01) and with two of the traits contributing to the first axis (with Nshoot, r = 0·70, P < 0·001; and with RDMC, r = 0·38, P < 0·001). The SRR, which reflects the partitioning of biomass to above- vs. below-ground plant parts, was not correlated with any of the other traits.
Fig. 2.
Principal component analysis (PCA) based on functional traits measured for ten replicates (in grey) of species used in the study (multivariate mean indicated in red). SRL, specific root length; SRR, shoot:root ratio; SLA, specific leaf area; LDMC, leaf dry matter content; Nshoot and Nroot, nitrogen concentration in the shoot and root, respectively. For D. glomerata, the site of origin is indicated in parentheses. Total variance explained by the two first axes: 62.93 %. Loading scores for the two first axis of the PCA for leaf and root traits are given in Supplementary Data Table S2.
Kinetic parameters of and uptake
The Imax values expressed per mass unit of root mass and unit of root length were strongly correlated (r = 0·86, P < 0·001); therefore, we only present Imax expressed on a mass basis. The Imax for both and differed between species (F-ratio = 6·02 and 3·90, P-value <0·001 and <0·01, respectively, Fig. 3A, B). The Imax values for all species were higher for (26–110 μmol h–1 g–1) than for (3–27 μmol h–1 g–1).
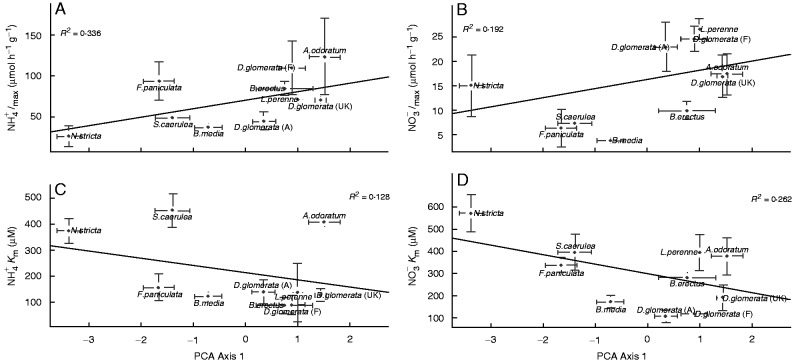
Fig. 3.
Relationships between uptake parameters. Maximum uptake rate per gram of dry root mass for (A) and (B) , affinity for (C) and (D) and the co-ordinates of individuals along the first axis of the PCA. R2 values for significant linear regression between variables (P-value <0.05) are indicated.
The Km reflects the ability of species to exploit N resources when their availability is very low, lower Km values indicating higher affinity for the substrate. The Km for both N forms differed between species (F-ratio = 6·53 and 5·8, P-value <0·001 for and , respectively, Fig. 3C, D). Dactylic glomerata (from all sites) had low Km values for both N forms, while N. stricta, A. odoratum and S. caerulea had high values for both forms (Table 1). Km values for both N forms were correlated (r = 0·70, P < 0·05).
More exploitative species such as D. glomerata had a higher Imax and lower Km (higher affinity) for both N forms compared with more conservative species such as N. stricta and S. caerulea. The hypothesis of a negative correlation between Km and Imax was tested, but no relationship was found for or for (, r = –0·12 n.s.; , r = –0·16 n.s.). Indeed, although this pattern held for some species, other species such as A. odoratum or B. media had high or low values, respectively, for both uptake parameters studied.
In order to investigate the relationships between uptake ability and resource use-strategies, as defined by functional traits, we analysed the relationship between kinetic parameters and species co-ordinates in the first axis of the PCA, which was identified as describing a resource-use strategy continuum from conservative to exploitative species. For both N forms, Imax was positively correlated with the PCA first axis, meaning higher N uptake for exploitative species (Fig. 3). and Km values were negatively correlated with the PCA axis, meaning that the exploitative species had higher affinity for and . All these relationships were also significant when the site of origin was taken into account (Supplementary Data Table S1). The specific examination of relationships between kinetic parameters and individual plant traits LDMC and SLA revealed that they appeared to be the more closely related, particularly with Km (Table 2).
Table 2.
Pearson correlation between the species mean (n = 10) for functional traits measured for leaves (SLA, LDMC, Nshoot), roots (SRL, RDMC, Nroot) and N uptake parameters (Imax and Km for and )
| SLA | LDMC | Nshoot | SRL | RDMC | Nroot | srr | Imax | Imax | Km | Km | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SLA | – | ||||||||||
| LDMC | –0·89 | – | |||||||||
| Nshoot | 0·77 | –0·80 | – | ||||||||
| SRL | –0·10 | –0·12 | –0·08 | – | |||||||
| RDMC | –0·63 | 0·43 | –0·68 | 0·26 | – | ||||||
| Nroot | 0·24 | –0·35 | 0·67 | 0·47 | –0·37 | – | |||||
| SRR | –0·03 | 0·09 | –0·15 | 0·44 | –0·02 | 0·21 | – | ||||
| Imax | 0·58 | –0·49 | 0·44 | 0·46 | –0·32 | 0·55 | 0·58 | – | |||
| Imax | 0·62 | –0·52 | 0·52 | –0·34 | –0·38 | 0·01 | –0·25 | 0·25 | – | ||
| Km | –0·38 | 0·68 | –0·39 | –0·47 | –0·07 | –0·27 | 0·34 | –0·12 | –0·20 | – | |
| Km | –0·68 | 0·82 | –0·50 | –0·18 | 0·17 | –0·11 | 0·19 | –0·17 | –0·16 | 0·70 | – |
P < 0·05 is indicated by bold; P < 0·1 is indicated by italics. Other values are not significant.
DISCUSSION
Relationships between root and leaf functional traits
The set of species in our study covered a representative range for herbaceous species for leaf functional traits involved in the leaf economics spectrum, spanning from more conservative to more exploitative (Cornelissen et al., 2003; Diaz et al., 2004; Wright et al., 2004). Conservative species such as S. caerula or N. stricta were characterized by low SLA and Nshoot, and high LDMC, indicating a low rate of tissue turnover adapted to nutrient-poor environments (Reich et al., 1992; Wilson et al., 1999). At the other extreme of the range, high SLA and low LDMC observed for D. glomerata, were consistent with an exploitative strategy typical of nutrient-rich sites (Garnier et al., 2001; Hodgson et al., 2011). Functional root traits are increasingly being included in the resource economics spectrum (Mommer and Weemstra, 2012), and our results give some support to this. Functional root traits such as RDMC and Nroot contributed significantly to the axis of specialization of species from conservation to exploitation. However, except for RDMC, root traits in our study were poorly correlated with leaf functional traits. Despite the lack of relationships between leaf and root traits, parallel contributions of leaf and root traits to the plant economics spectrum were apparent for the eight investigated grass species, as well as in other studies reporting similar functional syndromes despite various degrees of relationships between functional traits (Tjoelker et al., 2005; Roumet et al., 2006; Freschet et al., 2010, Fort et al., 2013).
Analogous functional traits for leaves and roots have already been related to similar plant features above- and below-ground, such as dry matter content to tissue longevity (Withington et al., 2006; McCormack et al., 2012), or N content to respiration rate (Reich et al., 1998; Tjoelker et al., 2005). However, we observed a second axis of differentiation between species involving variations in SRL and SRR, two traits that were poorly correlated with leaf traits or with the first axis. High SRL and low SRR are often associated with higher N uptake for exploitative species (Eissenstat, 1992; Reich et al., 1998; Ryser, 1998). However, the generality of the importance of those root traits in the exploitative strategy is still unknown since either positive relationships or absence of relationships have been reported (Craine and Lee, 2003; Tjoelker et al., 2005, respectively) between SRL and SLA, the analogous leaf trait for light capture (Wright et al., 2004). Different traits involved in the capture of different resources could be hypothesized to diverge if access to the resources differ. For example, leaf and root traits are more likely to co-vary if species compete both for light and for soil nutrients (e.g. Craine and Lee, 2003) than if access to the resources is more unrelated due to different environmental conditions (Chen et al., 2013; Freschet et al., 2013). Since root traits strongly depend on abiotic soil conditions (Robinson and Rorinson, 1988; Hodge, 2004), hydroponic conditions such as in our study, removing the physical constraints on root growth (Craine and Lee, 2003; Freschet et al., 2013), are a possible explanation for the lack of relationship between SLA and SRL. Also, having easy access to large amounts of nutrients could explain why N concentrations were observed to be well correlated between leaves and roots, while a trait related to the morphology such as SRL may be more important for access to the resource, which is less a problem for plants in hydropony. Overall, in spite of a low degree of correlation with leaf traits, our results highlighted that root traits are likely to contribute to the plant economics spectrum (Craine et al., 2005; Tjoelker et al., 2005; Roumet et al., 2006; Freschet et al., 2010; Reich, 2014).
Relationships between functional traits and N uptake
The exploitative strategy involves morphological and physiological traits such as high SLA and photosynthetic N-use efficiency (Poorter et al., 1990; Reich et al., 1999; Wright et al., 2001), which should be associated with higher ability to capture nutrients in response to higher N soil availability. While N acquisition has been demonstrated to be an important feature associated with this response (Osone et al., 2008; James et al., 2009), very few studies have investigated the relationships between functional traits involved in the resource economics spectrum and root N uptake, especially under controlled conditions that remove confounding effects of temperature, pH or N availability on N uptake (Louahlia et al., 1999; Jumpponen et al., 2002; Warren, 2009). Our results highlighted higher and uptake ability for more exploitative strategy species (Maire et al., 2009), as well as higher affinity for both N forms. Surprisingly, the best predictors of N uptake and affinity for and were two leaf structural traits, SLA and LDMC, rather than analogous root traits describing access to soil resources, such as SRL. Also, root or leaf N concentrations were poorly related to N uptake, supporting the idea that plant N concentration does not provide a direct indicator of the ability of species to take up N, as is commonly assumed, since other plant traits also influence N uptake (James, 2008). Although leaf and root traits were only weakly correlated in our experiment, leaf functional traits still appeared to be good functional markers of plant functioning (Garnier et al., 2004), with our results indicating higher uptake ability for species with functional traits indicative of a more exploitative strategy.
Our results also have intriguing implications for mechanisms of community assembly. Niche partitioning for different N forms has been proposed as a mechanism allowing species coexistence (Tilman, 1994; Miller and Bowman, 2002; Harrison et al., 2007). While there is some field evidence for preferential use of different N forms by coexisting plants (Ashton et al., 2010; but see Harrison et al., 2007), and that such specialization could be expressed as a trade-off in the uptake of and between exploitative and conservative species, respectively (Maire et al., 2009), our results do not support such findings. The uptake rates for and were not significantly correlated, and we even observed a positive relationship between affinities for the two N forms. We nevertheless acknowledge that our study was not specifically designed to deal with how N uptake strategies contribute to species coexistence. Furthermore, numerous field studies highlighted that N uptake of species is strongly influenced by soil N availability (Houlton et al., 2007; Stahl et al., 2011; Wang and Macko, 2011), which is likely to differ between habitats. However, using different species in similar conditions, especially in terms of N availability, we demonstrated that species N uptake ability was related to their functional traits, and consequently to their resource-use strategy. Further, we observed higher affinity for and for species with a more exploitative strategy, a result that contradicts the assumption that high affinity could be an attribute of species occurring under low nutrient availability (Lambers and Poorter, 1992; Näsholm et al., 2000) such as a conservative species. However, more investigations are needed to draw firm conclusions about these relationships. Indeed, while our experiment demonstrated that species with a more exploitative strategy had higher N uptake ability and affinity in near optimal conditions (hydropony), there is increasing evidence that both N preferences and N uptake ability of species depending on N availability in natural conditions are important determinants of species distributions (Maire et al., 2009; Ashton et al., 2010; Andersen and Turner, 2013). Therefore, future studies are needed to test whether the distinct patterns of N uptake between exploitative and conservative species observed in our study for a large set of species under controlled conditions exist in the field across gradients of soil fertility.
Conclusions
Using eight grass species covering a broad range of leaf trait values under controlled conditions, we highlighted that not only leaf functional traits but also a root trait, RDMC, contribute to the resource economics spectrum distinguishing conservative and exploitative strategies. Moreover, we observed higher root N uptake rates and affinity for exploitative species, suggesting greater ability to take up N from soil under a large range of N availability levels. Future studies should test whether the distinct patterns of N uptake between exploitative and conservative species observed under controlled conditions is also confirmed across gradients of soil fertility under natural conditions.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Table S1: linear mixed model with Imax and Km for ammonium and nitrate as response variables, and the first axis of the PCA and site as explanatory variables. Table S2: loading scores along the two first axes of the PCA for leaf and root traits.
ACKNOWLEDGEMENTS
We wish to thank Anne-Françoise Ameline and Anne-Sophie Desfeux for assistance during plant culture, and Marie-Paule Bataille and Raphael Segura for IRMS analysis. The authors thank the ‘Conseil Régional de Basse-Normandie’ for the funding of a post-doctoral position for F.G. This study was conducted as part of the ERA-Net BiodivERsA project VITAL, ANR-08-BDVA-008.
LITERATURE CITED
- Andersen KM, Turner BL. 2013. Preferences or plasticity in nitrogen acquisition by understorey palms in a tropical montane forest. Journal of Ecology 101: 819–825. [Google Scholar]
- Ashton IW, Miller AE, Bowman WD, Suding KN. 2010. Niche complementarity due to plasticity in resource use: plant partitioning of chemical N forms. Ecology 91: 3252–3260. [DOI] [PubMed] [Google Scholar]
- Bahn M, Knapp M, Garajova Z, Pfahringer N, Cernusca A. 2006. Root respiration in temperate mountain grasslands differing in land use. Global Change Biology 12: 995-1006. [Google Scholar]
- Bassirirad H. 2000. Kinetics of nutrient uptake by roots: responses to global change. New Phytologist 147: 155–169. [Google Scholar]
- Birouste M, Kazakou E, Blanchard A, Roumet C. 2012. Plant traits and decomposition: are the relationships for roots comparable to those for leaves? Annals of Botany 109: 463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom AJ. 1985. Wild and cultivated barleys show similar affinities for mineral nitrogen. Oecologia 65: 555–557. [DOI] [PubMed] [Google Scholar]
- Chapin FS. 1980. The mineral nutrition of wild plants. Annual Review of Ecology and Systematics 11: 233–260. [Google Scholar]
- Chen W, Zeng H, Eissenstat DM, Guo D. 2013. Variation of first-order root traits across climatic gradients and evolutionary trends in geological time. Global Ecology and Biogeography 22: 846–856. [Google Scholar]
- Chessel D, Dufour AB, Thioulouse J. 2004. The ade4 package-I. One-table methods . [Google Scholar]
- Comas LH, Eissenstat DM. 2004. Linking fine root traits to maximum potential growth rate among 11 mature temperate tree species. Functional Ecology 18: 388–397. [Google Scholar]
- Cornelissen JHC, Lavorel S, Garnier E, et al. 2003. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Australian Journal of Botany 51: 335–380. [Google Scholar]
- Craine JM, Lee WG. 2003. Covariation in leaf and root traits for native and non-native grasses along an altitudinal gradient in New Zealand. Oecologia 134: 471–478. [DOI] [PubMed] [Google Scholar]
- Craine JM, Lee WG, Bond WJ, Williams RJ, Johnson LC. 2005. Environmental constraints on a global relationship among leaf and root traits of grasses. Ecology 86: 12–19. [Google Scholar]
- Diaz S, Hodgson JG, Thompson K, et al. 2004. The plant traits that drive ecosystems: evidence from three continents. Journal of Vegetation Science 15: 295–304. [Google Scholar]
- Eissenstat DM. 1992. Costs and benefits of constructing roots of small diameter. Journal of Plant Nutrition 15: 763–782. [Google Scholar]
- Engels C, Neumann G, Gahoonia TS, George E, Schenk MK. 2000. Assessing the ability of roots for nutrients acquisition. In: Smit AL, Bengough AG, Engels C, van Noordwijk M, Pellerin S, van de Geijn SC, eds. Root methods. A handbook . Berlin: Springer, 403–460. [Google Scholar]
- Fort F, Jouany C, Cruz P. 2013. Root and leaf functional trait relations in Poaceae species: implications of differing resource-acquisition strategies. Journal of Plant Ecology 6: 211–219. [Google Scholar]
- Fortunel C, Fine PVA, Baraloto C. 2012. Leaf, stem and root tissue strategies across 758 Neotropical tree species. Functional Ecology 26: 1153–1161. [Google Scholar]
- Freijsen AHJ, Otten H. 1984. The effect of nitrate concentration in a flowing solution system on growth and nitrate uptake of two Plantago species. Plant and Soil 77: 159–169. [Google Scholar]
- Freschet GT, Bellingham PJ, Lyver PO, Bonner KI, Wardle DA. 2013. Plasticity in above- and belowground resource acquisition traits in response to single and multiple environmental factors in three tree species. Ecology and Evolution 3: 1065–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freschet GT, Cornelissen JHC, Van Logtestijn RSP, Aerts R. 2010. Evidence of the ‘plant economics spectrum’ in a subarctic flora. Journal of Ecology 98: 362–373. [Google Scholar]
- Garnier E. 1991. Resource capture, biomass allocation and growth in herbaceous plants. Trends in Ecology and Evolution 6: 126–131. [DOI] [PubMed] [Google Scholar]
- Garnier E, Koch GW, Roy J, Mooney HA. 1989. Responses of wild plants to nitrate availability. Oecologia 79: 542–550. [DOI] [PubMed] [Google Scholar]
- Garnier E, Shipley B, Roumet C, Laurent G. 2001. A standardized protocol for the determination of specific leaf area and leaf dry matter content. Functional Ecology 15: 688–695. [Google Scholar]
- Garnier E, Cortez J, Billès G, et al. 2004. Plant functional markers capture ecosystem properties during secondary succession. Ecology 85: 2630–2637. [Google Scholar]
- Glass AD. 2000. Homeostatic processes for the optimization of nutrient absorption: physiology and molecular biology. In BassiriRad H, ed. Nutrient acquisition by plants: an ecological perspective. Berlin: Springer, 117–140. [Google Scholar]
- Grigulis K, Lavorel S, Krainer U, et al. 2013. Relative contributions of plant traits and soil microbial properties to mountain grassland ecosystem services. Journal of Ecology 101: 47–57. [Google Scholar]
- Grime JP. 1977. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. American Naturalist 111: 1169–1194. [Google Scholar]
- Harrison KA, Bol R, Bardgett RD. 2007. Preferences for different nitrogen forms by coexisting plant species and soil microbes. Ecology 88: 989–999. [DOI] [PubMed] [Google Scholar]
- Hodge A. 2004. The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytologist 162: 9–24. [Google Scholar]
- Hodgson JG, Montserrat-Martí G, Charles M, et al. 2011. Is leaf dry matter content a better predictor of soil fertility than specific leaf area? Annals of Botany 108: 1337–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlton BZ, Sigman DM, Schuur EAG, Hedin LO. 2007. A climate-driven switch in plant nitrogen acquisition within tropical forest communities. Proceedings of the National Academy of Sciences, USA 104: 8902–8906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James JJ. 2008. Leaf nitrogen productivity as a mechanism driving the success of invasive annual grasses under low and high nitrogen supply. Journal of Arid Environments 72: 1775–1784. [Google Scholar]
- James JJ, Mangold JM, Sheley RL, Svejcar T. 2009. Root plasticity of native and invasive Great Basin species in response to soil nitrogen heterogeneity. Plant Ecology 202: 211–220. [Google Scholar]
- Jumpponen A, Högberg P, Huss-Danell K, Mulder CPH. 2002. Interspecific and spatial differences in nitrogen uptake in monocultures and two-species mixtures in north European grasslands. Functional Ecology 16: 454–461. [Google Scholar]
- Kronzucker HJ, Glass ADM, Siddiqi MY, Kirk GJD. 2000. Comparative kinetic analysis of ammonium and nitrate acquisition by tropical lowland rice: implications for rice cultivation and yield potential. New Phytologist 145: 471–476. [DOI] [PubMed] [Google Scholar]
- Lambers H, Poorter H. 1992. Inherent variation in growth rate between higher plants: a search for physiological causes and ecological consequences. In: Begon M, Fitter AH, eds. Advances in ecological research. New York: Academic Press, 187–261. [Google Scholar]
- Leffler AJ, James JJ, Monaco TA. 2013. Temperature and functional traits influence differences in nitrogen uptake capacity between native and invasive grasses. Oecologia 171: 51–60. [DOI] [PubMed] [Google Scholar]
- Liu G, Freschet GT, Pan X, Cornelissen JHC, Li Y, Dong M. 2010. Coordinated variation in leaf and root traits across multiple spatial scales in Chinese semi-arid and arid ecosystems. New Phytologist 188: 543–553. [DOI] [PubMed] [Google Scholar]
- Louahlia S, Macduff JH, Ourry A, Humphreys M, Boucaud J. 1999. Nitrogen reserve status affects the dynamics of nitrogen remobilization and mineral nitrogen uptake during recovery of contrasting cultivars of Lolium perenne from defoliation. New Phytologist 142: 451–462. [Google Scholar]
- Maire V, Gross N, Da Silveira Pontes L, Picon-Cochard C, Soussana J. 2009. Trade-off between root nitrogen acquisition and shoot nitrogen utilization across 13 co-occurring pasture grass species. Functional Ecology 23: 668–679. [Google Scholar]
- Makita N, Kosugi Y, Dannoura M, et al. 2012. Patterns of root respiration rates and morphological traits in 13 tree species in a tropical forest. Tree Physiology 32: 303–312. [DOI] [PubMed] [Google Scholar]
- McCormack L, Adams TS, Smithwick EAH, Eissenstat DM. 2012. Predicting fine root lifespan from plant functional traits in temperate trees. New Phytologist 195: 823–831. [DOI] [PubMed] [Google Scholar]
- Miller A, Bowman W. 2002. Variation in nitrogen-15 natural abundance and nitrogen uptake traits among co-occurring alpine species: do species partition by nitrogen form? Oecologia 130: 609–616. [DOI] [PubMed] [Google Scholar]
- Min X, Siddiqi MY, Guy RD, Glass ADM, Kronzucker HJ. 2000. A comparative kinetic analysis of nitrate and ammonium influx in two early-successional tree species of temperate and boreal forest ecosystems. Plant, Cell and Environment 23: 321–328. [Google Scholar]
- Mommer L, Weemstra M. 2012. The role of roots in the resource economics spectrum. New Phytologist 195: 725–727. [DOI] [PubMed] [Google Scholar]
- Muller B, Garnier E. 1990. Components of relative growth rate and sensitivity to nitrogen availability in annual and perennial species of Bromus. Oecologia 84: 513–518. [DOI] [PubMed] [Google Scholar]
- Näsholm T, Huss-Danell K, Högberg P. 2000. Uptake of organic nitrogen in the field by four agriculturally important plant species. Ecology 81: 1155–1161. [Google Scholar]
- Oscarson P, Ingemarsson B, Larsson C-M. 1989. Growth and nitrate uptake properties of plants grown at different relative rates of nitrogen supply. II. Activity and affinity of the nitrate uptake system in Pisum and Lemna in relation to nitrogen availability and nitrogen demand. Plant, Cell and Environment 12: 787–794. [Google Scholar]
- Osone Y, Ishida A, Tateno M. 2008. Correlation between relative growth rate and specific leaf area requires associations of specific leaf area with nitrogen absorption rate of roots. New Phytologist 179: 417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorter H, De Jong R. 1999. A comparison of specific leaf area, chemical composition and leaf construction costs of field plants from 15 habitats differing in productivity. New Phytologist 143: 163–176. [Google Scholar]
- Poorter H, Remkes C, Lambers H. 1990. Carbon and nitrogen economy of 24 wild species differing in relative growth rate. Plant Physiology 94: 621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. 2012. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Reich PB. 2014. The world-wide ‘fast–slow’ plant economics spectrum: a traits manifesto. Journal of Ecology 102: 275–301. [Google Scholar]
- Reich PB, Walters MB, Ellsworth DS. 1992. Leaf life-span in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecological Monographs 62: 365–392. [Google Scholar]
- Reich PB, Walters MB, Tjoelker MG, Vanderklein D, Buschena C. 1998. Photosynthesis and respiration rates depend on leaf and root morphology and nitrogen concentration in nine boreal tree species differing in relative growth rate. Functional Ecology 12: 395–405. [Google Scholar]
- Reich PB, Ellsworth DS, Walters MB, et al. 1999. Generality of leaf trait relationships: a test across six biomes. Ecology 80: 1955–1969. [Google Scholar]
- Robinson D, Rorison IH. 1988. Plasticity in grass species in relation to nitrogen supply. Functional Ecology 2: 249–257. [Google Scholar]
- Roumet C, Urcelay C, Díaz S. 2006. Suites of root traits differ between annual and perennial species growing in the field. New Phytologist 170: 357–368. [DOI] [PubMed] [Google Scholar]
- Ryser P. 1998. Intra- and interspecific variation in root length, root turnover and the underlying parameters. In: Čatský J, ed. Inherent variation in plant growth. Physiological mechanisms and ecological consequences. Leiden, The Netherlands: Backhuys Publishers, 441–465. [Google Scholar]
- Ryser P, Urbas P. 2000. Ecological significance of leaf life span among Central European grass species. Oikos 91: 41–50. [Google Scholar]
- Stahl VM, Beyschlag W, Werner C. 2011. Dynamic niche sharing in dry acidic grasslands – a 15N-labeling experiment. Plant and Soil 344: 389–400. [Google Scholar]
- Tilman D. 1990. Constraints and tradeoffs: toward a predictive theory of competition and succession. Oikos 58: 3–15. [Google Scholar]
- Tilman D. 1994. Competition and biodiversity in spatially structured habitats. Ecology 75: 2–16. [Google Scholar]
- Tjoelker MG, Craine JM, Wedin D, Reich PB, Tilman D. 2005. Linking leaf and root trait syndromes among 39 grassland and savannah species. New Phytologist 167: 493–508. [DOI] [PubMed] [Google Scholar]
- Wang L, Macko SA. 2011. Constrained preferences in nitrogen uptake across plant species and environments. Plant, Cell and Environment 34: 525–534. [DOI] [PubMed] [Google Scholar]
- Warren CR. 2009. Why does temperature affect relative uptake rates of nitrate, ammonium and glycine: a test with Eucalyptus pauciflora. Soil Biology and Biochemistry 41: 778–784. [Google Scholar]
- Weiher E, van der Werf A, Thompson K, Roderick M, Garnier E, Eriksson O. 1999. Challenging Theophrastus: a common core list of plant traits for functional ecology. Journal of Vegetation Science 10: 609–620. [Google Scholar]
- Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ. 2002. Plant ecological strategies: some leading dimensions of variation between species. Annual Review of Ecology and Systematics 33: 125–159. [Google Scholar]
- Wilson CA, Mitchell RJ, Hendricks JJ, Boring LR. 1999. Patterns and controls of ecosystem function in longleaf pine – wiregrass savannas. II. Nitrogen dynamics. Canadian Journal of Forest Research 29: 752–760. [Google Scholar]
- Withington JM, Reich PB, Oleksyn J, Eissenstat DM. 2006. Comparisons of structure and life span in roots and leaves among temperate trees. Ecological Monographs 76: 381–397. [Google Scholar]
- Wright IJ, Reich PB, Westoby M. 2001. Strategy shifts in leaf physiology, structure and nutrient content between species of high- and low-rainfall and high- and low-nutrient habitats. Functional Ecology 15: 423–434. [Google Scholar]
- Wright IJ, Reich PB, Westoby M, et al. 2004. The worldwide leaf economics spectrum. Nature 428: 821–827. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.