Abstract
Objective
The study documents whether socioeconomic status (SES) differentials in biological risk are more widely observed and larger in the United States than Taiwan.
Method
Data come from the Social Environment and Biomarkers of Aging Study in Taiwan and the Midlife in the United States study. We use regression analyses to test whether four summary measures of biological risk are significantly related to categorical measures of education, income, and subjective social status among four country-sex specific subgroups.
Results
Physiological dysregulation is significantly, negatively related to SES in both the US and Taiwan, especially for males. The prevalence and magnitude of the relationships are similar in the two countries:12 of 24 possible SES-biological summary score relationships are significant in the US and 11 of 24 are significant in Taiwan.
Discussion
Overall, SES differentials in biological risk do not appear to be more widely observed or larger in the US than in Taiwan.
Keywords: socioeconomic status, biological markers, physiological dysregulation, United States, Taiwan
INTRODUCTION
Even though the United States spends more money -- both as a percentage of Gross Domestic Product and per capita -- on health care than other high-income countries (Squires, 2011), the US lags behind other high-income countries in many areas of health (National Research Council & Institute of Medicine, 2013). For instance, in a study of 17 high-income countries with comparable data in the Human Mortality Database, the US has the second highest mortality rate from communicable diseases and the 4th highest rate from non-communicable diseases (Figure 1-1 and Figure 1-2, National Research Council & Institute of Medicine, 2013, p. 26-27). Life expectancy at birth lags for the US, with US males ranking the lowest among the 17 countries and US females ranking the second lowest (National Research Council & Institute of Medicine, 2013, Table 1, p. 39). Evidence also indicates that compared with many European countries, Canada, Japan, and England, adults age 50 and over in the US have higher rates of conditions such as hypertension, heart disease, stroke, lung disease, cancer, and high cholesterol (Avendano, Glymour, Banks, & Mackenbach, 2009; Banks & Smith 2011; Tables 3-2, 3-3 & 3-5 & Figure 3-4 in Crimmins, Garcia, & Kim, 2010; Thorpe, Howard, & Galactionova, 2007).
Although data suggest that the US health disadvantage persists across all levels of socioeconomic status (SES), it is most pronounced among those with the lowest SES, resulting in larger gaps in health between individuals with high versus low SES than in other countries. In a comparison with England and the 10 countries in the European Longitudinal Study of Aging, the wealthiest Americans had worse health than their wealthy English or European counterparts, but the differences were even bigger for the least wealthy Americans compared with the least wealthy English and Europeans (Avendano et al., 2009). Several studies have described similar relationships for education and income: Americans fared worse than the English and most Europeans at all levels of income and education, but the health and mortality gaps were largest at the lowest levels of education and income (Avendano et al., 2009; Avendano et al., 2011; Banks, Marmot, Oldfield, & Smith, 2006). This excess morbidity and mortality at the lower end of the SES hierarchy, which is also reflected in steeper negative health gradients in the US (Banks et al., 2006), may be one reason for the poor overall health ranking of the US (National Research Council & Institute of Medicine, 2013).
The recent reports by the National Research Council & Institute of Medicine (2013) and the National Research Council (2011) propose a number of potential reasons for the larger SES gaps in health and mortality in the US. For example, compared with many other countries that have universal or nearly universal health care coverage, access to quality care is more restricted in the US and significant portions of the population, particularly low SES individuals, do not have health insurance. Negative individual health behaviors, such as poor diet, low physical activity, and exposure to violence, especially among individuals with low SES, are also more prevalent in the US than other countries. In addition, a relatively weaker social safety net (publically funded social programs) in the US may exacerbate the negative effects of low income, low education and unemployment on health whereas stronger public transfer systems in other countries may provide safeguards against such effects. Although debated in the literature, the degree of economic inequality in a society may also shape the SES-health relationship with SES disparities being more evident in societies where social inequalities are greater than in societies where inequalities are narrower. Countries with greater social inequality may exhibit greater status competition resulting in more insecurity across status groups and more mistrust between status groups (Wilkinson & Pickett 2006; Wilkinson & Pickett 2007). As suggested by these recent reports (National Research Council, 2011; National Research Council and Institute of Medicine, 2013), these factors could operate to put lower SES individuals in the US not only at a greater health disadvantage compared with higher SES individuals in the US, but also in a worse position compared with lower SES individuals in other high-income countries.
Crimmins & Seeman (2004) note that social and economic circumstances, such as those mentioned above, work primarily through biological factors to affect health: Elevated biological risk is the precursor to the development of diseases and loss of bodily functions, which are then followed by frailty, and ultimately death (Crimmins, Kim & Vasunilashorn, 2010; Crimmins & Seeman 2004). These biological factors, in turn, reflect the effects on the body of living and working in different social and economic conditions (Crimmins, Kim & Vasunilashorn, 2010; Goldman & Dowd, 2009; McEwen & Seeman, 1999). Understanding SES differentials in biological risk, therefore, may provide insights for better understanding patterns of social disparities in health (Crimmins, Kim & Vasunilashorn, 2010).
In this study we compare SES differentials in biological markers of risk for poor health among older men and women in the United States and Taiwan. International comparisons allow us to explore possible explanations for cross-country differences that emerge (Banks et al., 2006). Taiwan serves as an interesting comparison for a number of reasons. First, Taiwan is similar to other, primarily European, countries that have been compared to the US; It has lower income inequality than the US1 and a near universal health care system (Ofstedal et al., 2002). It also differs from comparison countries in the structure of its social safety net, which focuses more on family support than on government sponsored programs. Taiwan’s expenditures on social welfare programs are relatively limited and narrowly focused (Huang and Ku, 2011), so the social safety net relies heavily on strong norms of filial responsibility and well-established expectations for intergenerational support (Cornman 1999).
A further unique feature of the comparison of Taiwan with the U.S. is that both samples used in the present investigation include a large number of comparable biological markers, including indicators of inflammation not always available in population-based studies. Previous studies of the SES-biological risk relationship show that the relationship can vary across different indicators of biological risk (e.g. Goldman, Turra, Rosero-Bixby, Weir & Crimmins, 2011; Rosario-Bixby & Dow 2009; Schooling et al., 2008). In addition, with a larger set of biological measures, we are able to combine biomarkers to examine SES differences not only in overall physiological dysregulation (PD; Seeman et al., 2004; Seeman et al., 2008) but also in subsets of biomarkers that reflect cardiovascular and metabolic risk (CV/metabolic), hypothalamic-pituitary-adrenal axis and sympathetic nervous system function (HPA/SNS), and inflammation. These composites are important because the effect of experiences captured by measures of SES may not be fully realized in any individual biomarker measure (Seeman et al., 2008).
Finally, with data from Taiwan, we are able to compare SES differentials using multiple measures of SES: education, income, and subjective social status-- a measure of perceived relative social standing. Comparing differences between such measures of SES and biological risk in a single study can provide insight into whether biomarker disparities stem from differences in actual material resources and/or from differences in more subjective factors, giving a fuller picture of the SES-biological risk intersection. Low income and education are thought to negatively affect health because they limit access to, opportunities for, and knowledge about health care and healthy behaviors (diet, exercise, smoking, etc.), as well as lead to increased exposures to disadvantageous or challenging situations at home, the workplace, neighborhoods, and communities (Crimmins & Seeman, 2004; National Research Council & Institute of Medicine, 2013). Prior studies have primarily focused on absolute or objective levels of SES, such as education and income, and have shown that in general, better health is associated with higher levels of socioeconomic status (see reviews of the literature in Adler & Ostrove, 1999; Elo, 2009), although findings regarding SES gradients in biological risk are less consistent (see for instance Dowd & Goldman, 2006; Schooling et al., 2008; Rosero-Bixby & Dow, 2009; Goldman et al., 2011).
In addition to absolute levels of SES, relative deprivation or low relative position in the social hierarchy also can be important in determining health (Eibner & Evans, 2005; Singh-Manoux, Marmot, & Adler 2005). The relative deprivation hypothesis suggests that having lower status, whether actual or perceived, than one’s reference group, may cause stress or negative emotions, which, in turn, may increase biological risk and negatively affect health (Eibner & Evans, 2005; Singh-Manoux, Marmot, & Adler 2005). Findings from both U.S. and Japanese samples have documented the adverse consequents of relative deprivation in income on health (Kondo, Kawachi, Subramanian, Takeda, & Yamagata, 2008; Subramanyam, Kawachi, Berkman, & Subramanian, 2009). Subjective social status, the measure of relative standing used in this study, has been shown to reflect a combination of objective measures of, and socio-cultural influences on, social position (Singh-Manoux, Adler, & Marmot, 2003; Wright & Steptoe, 2005; Franzini & Fernandez-Esquer, 2006; Goldman, Cornman, & Chang, 2006). Numerous studies find that lower actual or perceived position in the social hierarchy is negatively associated with health and/or biological risk (Eibner & Evans, 2005; Demakakos, Nazroo, Breeze, & Marmot, 2008; Singh-Manoux, Adler, & Marmot 2003; Singh-Manoux, Marmot, & Adler 2005; Adler, Epel, Castellazzo, Ickovicks, 2000; Chen, Covinsky, Stijacic Cenzer, Adler, & Williams, 2012).
Based on the literature reviewed here, we have formulated the following hypotheses. First, we expect all three measures of socioeconomic status to be negatively associated with biological risk. Second, we expect SES differentials to be more prevalent (i.e., we anticipate finding a greater number of significant relationships between the three SES measures and the scores of biological risk) in the US than in Taiwan. Finally, where SES is significantly associated with biological risk, differentials will be larger in the US than in Taiwan.
METHODS
Data
Data for these analyses came from two sources: 1) the second wave of the Social Environment and Biomarkers of Aging Study (SEBAS; Chang et al., 2012) in Taiwan, a follow-up study to the Taiwan Longitudinal Study of Aging (TLSA) that was first carried out in 1989 on a nationally representative sample of 4,049 persons age 60 and over, repeated approximately every three years with refreshed samples; and 2) the second wave of the Midlife in the United States study (MIDUS; Ryff et al., 2007; Ryff, Seeman, & Weinstein, 2013), a follow-up conducted in 2004-2005 (9 to 10 years after the first wave). Our analyses focused on the second wave of SEBAS because it included a more extensive set of biological markers than the first wave (conducted in 2000) and on the second wave of MIDUS because the first wave (conducted in 1995-96) did not include any biomarker measures.
The second wave of SEBAS, conducted in 2006, included the surviving exam participants from SEBAS 2000 and a randomly selected subset of younger respondents who entered the TLSA in 2003, resulting in a nationally representative sample of adults aged 53 and over. Respondents were interviewed in their homes and participated in a hospital-based physical examination. The exam occurred several weeks after the home-interview in a hospital near the respondents’ homes and included the collection of a fasting blood sample and measurement of height, weight, waist-hip circumference and blood pressure. Respondents also provided a 12-hour overnight (7pm-7am) urine sample that was brought to the hospital on the morning of the exam. Of the 1,481 respondents eligible to participate in SEBAS II, 1,284 respondents (86.7%) were interviewed in their homes, and 1,036 of these respondents (80.7%), aged 53-94, participated in the physical exam. The home-interview respondents who did not participate in the physical exam did not differ from exam participants in terms of age, sex, income, marital status, or self-assessed health (excellent, good, or average vs. not so good or poor), but the non-participants had slightly less education (average education was 6.2 vs. 6.9 years, p=0.03) and slightly lower subjective social status (average score was 4.1 vs. 4.3, p=0.03), which could result in an underestimation of education and subjective social status differences in biological risk. Among the 1,036 exam participants in SEBAS, we excluded 60 respondents who were missing data on one or more biomarkers, resulting in a final analytical sample of 976 respondents (526 males and 450 females). For more details about the study, see Chang, Glei, Goldman, & Weinstein (2007) and Chang et al. (2012).
The second wave of MIDUS included 4,936 respondents (75% of survivors from MIDUS I, now aged 34-84) who completed the telephone survey, 4,041 of whom also returned a mail-in self-administered questionnaire (SAQ; Radler & Ryff, 2010). Of the 3,191 eligible respondents from the main telephone follow-up (alive in 2004-05, sufficiently healthy for safe travel to a study clinic, and completed both the telephone interview and the SAQ), 1,255 participated in a two-day visit to a clinical research center for a physical exam (Love, Seeman, Weinstein, & Ryff, 2010). The exam included the collection of a fasting blood sample before breakfast on the second day of the respondent’s hospital stay; a 12-hour (7p.m. to 7 a.m.) urine sample that began on day 1; and measurement of height, weight, waist-hip circumference, and blood pressure (Love et al., 2010). Respondents participating in the biological protocol did not differ from the full follow-up sample in terms of age, sex, race, marital status, self-assessed health or income, but they were more likely to have a college degree and less likely to have only a high school education or some college (Love et al., 2010), potentially leading to an underestimation of differences in risk by education. To avoid biasing results, we excluded from the clinic sample 201 respondents who were part of an oversample of the African American population in Milwaukee, Wisconsin. We also excluded an additional 40 respondents who were missing one or more of the biomarker measures. The final analytical sample for the US, therefore, consisted of 1,014 respondents (460 males and 554 females). Additional study details can be obtained from Ryff, et al., (2007), Radler & Ryff (2010), and Love et al., (2010).
Measures
We constructed a summary score related to overall PD and three summary subscores reflecting CV/metabolic risk, HPA/SNS function, and inflammation. Construction of the summary scores was modeled after the physiological dysregulation score from a previous study (Glei, Goldman, Lin, & Weinstein, 2012), including only biological markers that were measured in both SEBAS II and MIDUS II. Each summary score was a count of the number of biological markers on which respondents scored in the high risk range, with high-risk derived from either established clinical cutoff points or the combined distributions of both countries. For distribution-based cutoffs, we used weighted distributions for SEBAS to account for oversampling of older respondents and urban residents.
The PD score included 20 biological markers. High risk for systolic and diastolic blood pressures (each being the average of the second and third (out of three) seated readings), total cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, glycosylated hemoglobin, body mass index (BMI), resting pulse rate, and high sensitivity C-reactive protein (CRP) was determined using previously published clinical cutoff points (Gruenwald, Seeman, Ryff, Karlamangla, & Singer, 2006; Jouven et al., 2005; National Cholesterol Education Program 2001; World Health Organization, 2013; Zimmet, George, Albertini, & Rios, 2005). There are no established high risk cutpoints for the remaining 11 biomarkers, so high risk was based on the pooled distribution of each indicator. For waist-hip ratio, urinary cortisol, norepinephrine, interleukin-6 (IL-6), fibrinogen, soluble intercellular adhesion molecule-1 (sICAM-1), sE-selectin, and soluble IL-6 receptor (sIL-6R), high risk was determined by whether a biomarker value was greater than or equal to the 80th percentile. High risk for dehydroepiandrosterone-sulfate (DHEA-S) and creatinine (used to calculate creatinine clearance) was indicated by having a biomarker value less than or equal to the 20th percentile. An indication of high risk for epinephrine was having a biomarker value in the top or bottom decile of the pooled distribution (Glei et al., 2012). See Supplemental Table 1 for the assay methods used to ascertain individual biomarkers and Supplemental Table 2 for high-risk cut-off values for each biomarker.
The CV/metabolic subscore represented a count of the number of nine cardiovascular markers that were high risk (systolic and diastolic blood pressure, total cholesterol, HDL cholesterol, triglycerides, glycosylated hemoglobin, BMI, waist-hip ratio, and resting pulse). The four markers included in the HPA/SNS subscore were epinephrine, norepinephrine, DHEAS, and urinary cortisol. Finally, the inflammation subscore comprised six biomarkers: serum IL-6, CRP, fibrinogen, sICAM-1, sE-selectin, and sIL-6R.
Analyses incorporated three measures of SES: country-specific education categories, income quartiles, and subjective social status. In Taiwan, education was measured using the number of years of schooling completed, which ranged from 0 to 17 or more years. We examined differences across three broad sex-specific educational groups (Goldman et al., 2011): low (males: 0-5 years, females: 0 years), medium (males: 6 years, females:1-6 years), and high (7+ years for both males and females). For the US respondents, education was measured as the highest level of completed education. We collapsed responses into three educational categories, which were the same for both males and females: high school graduate or less (low), some college or vocational training (medium), and college degree or higher (high). Finally, we used variations of the MacArthur Scale of Subjective Social Status (Adler et al., 2009) implemented in SEBAS and MIDUS to capture respondents’ sense of their position in the social hierarchy. Respondents were shown a picture of a ladder with 10 rungs and told that the ladder represented where people stood in relation to each other in Taiwan (SEBAS) or their community (MIDUS) with the top of the ladder representing people who were the best off (SEBAS)/had the highest standing (MIDUS) and the bottom of the ladder representing people who were the worst off/had the lowest standing. Respondents were asked to place themselves on the ladder according to where they thought they belonged in the hierarchy. For both samples, ladder scores were collapsed into three categories with country-specific cutoffs for each category: low=scores less than the median; medium=scores equal to the median; and high=scores greater than the median.
Approximately 6 % of respondents in each study were missing information on one or more of the SES variables. For respondents missing data on education (n=2 MIDUS respondents) we imputed the data with the modal education level by age and sex. Missing data on income (n=35 SEBAS and 48 MIDUS respondents) were imputed based on the modal category by age, education, and sex. Finally, missing ladder information (n=26 SEBAS and 20 MIDUS respondents) was imputed based on the modal category by education, income, and sex. To test the sensitivity of our results to this treatment of missing data, we estimated the final models on two additional datasets: 1) missing data replaced with multiply imputed values (Marchenko and Royston 2009; StataCorp, 2009) and 2) only respondents with complete data. In both sets of analyses, results were nearly identical to those based on the sample with data imputed using modal replacement of missing data, which we present here.
Analyses included two additional control variables. We controlled for age (age and age-squared) to account for the age differences between the Taiwan and US samples and for race/ethnicity, which has been shown to be a confounding factor in analyses of SES and health in both the US (Braveman et al., 2005; Braveman, Cubbin, Egerter, Williams, & Pamuk, 2010; Williams & Sternthal, 2010) and Taiwan (Hermalin, Ofstedal, Sun & Llu, 2009). In the US, we contrasted non-white vs. white respondents and in Taiwan, Mainland Chinese vs. native Taiwanese (Hakka, Fukienese, Aboriginal).
Analysis
In separate models, each of the biological summary scores was regressed on each SES indicator controlling for age and race/ethnicity. Previous research has shown that the association between SES and health varies by sex (Dowd & Goldman, 2006). We therefore estimated models separately for males and females. Because the biological summary scores were count-type variables, we initially estimated both Poisson and negative binomial regression models. To determine which model was the better fit, we compared the models using a log-likelihood ratio test. We then examined additional tests and model fit statistics, including the Akaike information criterion (AIC), the Bayesian information criterion (BIC), and the Vuong test (StataCorp, 2009) to establish whether the regular or zero-inflated version of the Poisson or negative binomial model was preferred. (Model fit statistics and tests are available from the authors). After selecting the better model, we also tested the joint significance of the categories for a given SES variable in the model using a Wald test with a chi-square distribution (StataCorp, 2009). Finally, based on the final model and using the ‘margins’ command in Stata (StataCorp, 2009), we estimated the marginal means for each summary score for each level of each SES variable, calculated the difference in the means between the lowest and highest categories for each SES indicator, and graphed these differentials for the biological summary scores for which the joint significance test indicated significant SES differences.
RESULTS
Demographic and socioeconomic characteristics of the sample are shown in Table 1. Most SEBAS respondents were aged 55-74 and the mean age was 66 years for males and 65 years for females. The majority of MIDUS respondents were aged 45-64 with a mean age of 59 years for males and 58 years for females. Less than 15% of SEBAS respondents were Mainland Chinese and less than 10% of MIDUS respondents were non-white.
Table 1.
Demographic and socioeconomic status characteristics by sex and study
| SEBAS (Weighted %)a
|
MIDUS (%)
|
|||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| Age (in years) | ||||
| 35-44 | -- | -- | 12.2 | 14.4 |
| 45-54 | 8.7 | 8.5 | 26.7 | 29.1 |
| 55-64 | 40.4 | 49.4 | 30.4 | 29.4 |
| 65-74 | 30.9 | 27.1 | 17.6 | 19.7 |
| 75+ | 20.1 | 15.0 | 13.0 | 7.4 |
| (mean age) | 65.8 | 64.6 | 58.7 | 57.6 |
| Race/Ethnicity | ||||
| Non-White (vs. White) | -- | -- | 8.0 | 9.4 |
| Mainland Chinese (vs. Taiwanese) | 13.5 | 5.7 | -- | -- |
| Education b | ||||
| Low | 15.7 | 29.3 | 20.2 | 28.0 |
| Medium | 40.9 | 44.3 | 28.7 | 29.1 |
| High | 43.4 | 26.4 | 51.1 | 43.0 |
| Income quartiles c | ||||
| Q1 | 22.1 | 27.3 | 20.2 | 29.8 |
| Q2 | 24.5 | 27.1 | 25.7 | 22.7 |
| Q3 | 24.7 | 26.0 | 27.2 | 23.3 |
| Q4 | 28.7 | 19.5 | 27.0 | 24.2 |
| Ladder score d | ||||
| Low | 50.0 | 43.8 | 33.9 | 46.6 |
| Medium | 26.4 | 35.5 | 28.9 | 23.7 |
| High | 23.5 | 20.7 | 37.2 | 29.8 |
|
| ||||
| n | 526 | 450 | 460 | 554 |
We present weighted distributions for SEBAS to account for oversampling of older respondents and urban residents
SEBAS education categories: for males, low= 0-5 years, medium=6 years, and high= 7+ years; for females, low= 0 years, medium=1-6 years, and high=7+ years. MIDUS education categories are: high school or less, some college/2 yr degree, and 4-5 yr college degree or more.
Income quartiles SEBAS: Q1=NT$0 - NT$144,000; Q2=NT$145,500-NT$270,000; Q3=NT$271,500-NT$605,000; and Q4=NT$620,000-NT$860,000. Income quartiles MIDUS: Q1=$0-$39,000; Q2=$39,024 - $62,500; Q3 = $62,750-$101,250; and Q4 = $101,500-$400,000.
SEBAS ladder score categories: low=rungs 0-4; medium=rung 5; high=rungs 6-10. MIDUS. ladder score categories: low=rungs 0-6; medium=rung 7; high=rungs 8-10.
Table 2 shows age-adjusted descriptive statistics for the biomarker summary scores by sex and study. The observed range of the PD index was 0-13 (out of a possible 20) for SEBAS males and MIDUS males and females and was 0-14 for SEBAS females, with less than 4% of the population subgroups scoring zero. The age-adjusted mean score was about 5 for SEBAS males and females and MIDUS males and 4 for MIDUS females. The CV/metabolic risk subscore ranged from 0-7 (out of 9) for SEBAS males and females, 0-9 for MIDUS males and 0-8 for MIDUS females. The percent scoring zero ranged from about 6% (SEBAS females) to 13% (MIDUS females) and the age-adjusted mean ranged from 2.1 (MIDUS females) to 3.1 (MIDUS males). The HPA/SNS score ranged from 0-3 for MIDUS males and 0-4 for the other sex-country subgroups, with about 26% (SEBAS females) to 56% (SEBAS males) of respondents having zero high risk indicators. The mean HPA/SNS function score was less than 1.0 for each of the male populations and for MIDUS females and was about 1.0 for SEBAS females. Finally, between 35% (MIDUS females) and 43% (MIDUS males) of respondents were high risk on zero out of six inflammation indicators and the age-adjusted mean inflammation score ranged between 1.0 (MIDUS males) and 1.5 (SEBAS males).
Table 2.
Descriptive statistics for physiological dysregulation summary scores and subscores by sex and study
| Biomarker | Observed Rangea | % Scoring Zero | Age adjusted Mean | Standard error |
|---|---|---|---|---|
| SEBAS Males (n=526)b | ||||
| Overall Physiological Dysregulationc | 0-13 | 2.3 | 5.1 | 0.20 |
| CV/metabolic Subscore | 0-7 | 8.9 | 2.5 | 0.11 |
| HPA/SNS Function Subscore | 0-4 | 56.3 | 0.6 | 0.06 |
| Inflammation Subscore | 0-6 | 39.2 | 1.5 | 0.12 |
| SEBAS Females (n=450)b | ||||
| Overall Physiological Dysregulationc | 0-14 | 0.2 | 5.0 | 0.13 |
| CV/metabolic Subscore | 0-7 | 6.2 | 2.4 | 0.08 |
| HPA/SNS Function Subscore | 0-4 | 25.8 | 1.2 | 0.05 |
| Inflammation Subscore | 0-6 | 37.8 | 1.1 | 0.06 |
| MIDUS Males (n=460) | ||||
| Overall Physiological Dysregulationc | 0-13 | 2.8 | 4.7 | 0.17 |
| CV/metabolic Subscore | 0-9 | 7.2 | 3.1 | 0.13 |
| HPA/SNS Function Subscore | 0-3 | 53.7 | 0.5 | 0.03 |
| Inflammation Subscore | 0-6 | 42.6 | 1.0 | 0.07 |
| MIDUS Females (n=554) | ||||
| Overall Physiological Dysregulationc | 0-13 | 3.4 | 4.1 | 0.14 |
| CVD/metabolic Subscore | 0-8 | 13.4 | 2.1 | 0.09 |
| HPA/SNS Function Subscore | 0-4 | 35.0 | 0.7 | 0.04 |
| Inflammation Subscore | 0-6 | 34.7 | 1.2 | 0.07 |
The theoretical ranges of the summary scores are as follows: PD - 0 to 20; CV/metabolic – 0 to 9; HPA/SNS – 0 to 4; Inflammation – 0 to 6;
The SEBAS distributions are weighted to account for oversampling of older respondents and urban residents
One biomarker (creatinine clearance) is not included in any of the subscores.
Overall, regression results (Tables 3 and 4) showed that the relationships between biomarker summary scores and SES were in the expected direction: higher SES scores were associated with less biological risk, although not all relationships were statistically significant. Based on the chi-square tests of joint significance, the overall PD score was significantly and negatively associated with all three SES measures among males in Taiwan and with education and income among their US counterparts (Table 3), with differences lying primarily between respondents in the highest category of the SES variable and respondents in all other categories. The three summary subscores were less consistently associated with SES. The CV/metabolic score was not significantly related to any of the SES measures among Taiwanese males and only to education among US males (χ2 =19.03, 2 df, p<0.01) with higher levels of education associated with lower CV/metabolic risk. HPA/SNS function was associated with lower risk among both Taiwanese and US males with higher levels of education (χ2=9.69, 2 df, p<0.01 and χ2=7.84, 2 df, p<0.05, respectively), was not significantly related to subjective social status in either population, and was negatively associated with high income only among Taiwanese males (χ2=9.64, 3 df, p<0.05). Finally, the chi-square tests of joint significance indicated that the inflammation subscore was significantly and negatively associated with all three SES measures among both Taiwanese and US males.
Table 3.
Associations between socioeconomic status and biomarker scores of health (incident rate ratios: eb): Males
| Outcomes (Type of Model)
|
||||||||
|---|---|---|---|---|---|---|---|---|
| SEBAS Males | MIDUS Males | |||||||
|
|
|
|||||||
| PD (NB) | CV/metabolic (P) | HPA/SNS (P) | INF (ZINB) | PD (NB) | CV/metabolic (NB) | HPA/SNS (P) | INF (ZINB)d | |
| Education Model (includes age, age-squared, race/ethnicity, education a) | ||||||||
| (Low education omitted) | ||||||||
| Medium education | 0.95 | 0.95 | 1.10 | 0.90 | 0.94 | 0.94 | 0.91 | 0.97 |
| High education | 0.82** | 0.92 | 0.73 | 0.68** | 0.71** | 0.76** | 0.67* | 0.62** |
| p-value medium vs. high education | 0.007 | 0.586 | 0.002 | 0.007 | <0.001 | 0.001 | 0.042 | <0.001 |
| χ2 (2 df) | 10.77** | 1.02 | 9.69** | 11.39** | 34.92** | 19.03** | 7.84* | 19.21** |
| Income Models (includes age, age-squared, race/ethnicity, income quartiles b) | ||||||||
| (Q1 omitted) | ||||||||
| Q2 | 1.00 | 0.95 | 0.90 | 1.07 | 0.83* | 0.88 | 0.89 | 0.70* |
| Q3 | 0.91 | 0.94 | 0.92 | 0.83 | 0.96 | 0.97 | 1.09 | 0.91 |
| Q4 | 0.78** | 0.88 | 0.58** | 0.67** | 0.77** | 0.82* | 0.80 | 0.65** |
| p-value Q2 vs. Q3 | 0.176 | 0.893 | 0.885 | 0.038 | 0.052 | 0.238 | 0.247 | 0.085 |
| p-value Q3 vs. Q4 | 0.023 | 0.416 | 0.008 | 0.120 | 0.004 | 0.029 | 0.083 | 0.022 |
| χ2 (3 df) | 14.66** | 1.91 | 9.64* | 13.41** | 14.40** | 7.33 | 3.39 | 10.71* |
| Ladder Score Models (includes age, age-squared, race/ethnicity, ladder score c) | ||||||||
| (Low ladder score omitted) | ||||||||
| Middle ladder score | 0.92 | 0.97 | 0.81 | 0.85 | 0.91 | 1.00 | 0.87 | 0.72* |
| High ladder score | 0.84** | 0.93 | 0.78 | 0.73** | 0.90 | 0.96 | 0.93 | 0.73* |
| p-value for middle vs. high | 0.250 | 0.533 | 0.831 | 0.269 | 0.800 | 0.546 | 0.667 | 0.921 |
| χ2 (2 df) | 8.18* | 1.14 | 4.16 | 8.31* | 3.42 | 0.51 | 0.74 | 8.46* |
|
| ||||||||
| n | 526 | 460 | ||||||
Note. SEBAS = Social Environment and Biomarkers of Aging Study; MIDUS = Midlife in the United States; PD = physiological dysregulation; NB = negative binomial regression model; CV/metabolic = cardiovascular and metabolic function score; P = Poisson regression model; HPA/SNS = hypothalamic–pituitary–adrenal axis and sympathetic nervous system; INF = inflammation score; ZINB = zero-inflated negative binomial.
SEBAS education categories: for males, low= 0-5 years, medium=6 years, and high= 7+ years; for females, low= 0 years, medium=1-6 years, and high=7+ years. MIDUS education categories are: high school or less, some college/2 yr degree, and 4-5 yr college degree or more.
Income quartiles SEBAS: Q1=NT$0 - NT$144,000; Q2=NT$145,500-NT$270,000; Q3=NT$271,500-NT$605,000; and Q4=NT$620,000-NT$860,000. Income quartiles MIDUS: Q1=$0-$39,000; Q2=$39,024 - $62,500; Q3 = $62,750-$101,250; and Q4 = $101,500-$400,000. Note that 1 NT$ = 0.33 US$.
SEBAS ladder score categories: low=rungs 0-4; medium=rung 5; high=rungs 6-10. MIDUS ladder score categories: low=rungs 0-6; medium=rung 7; high=rungs 8-10.
The best fitting model for regressing the inflammation score on ladder score is the negative binomial regression model.
p < 0.05;
p<0.01
Table 4.
Associations between socioeconomic status and biomarker scores of health (incident rate ratios: eb): Females
| Outcomes (Type of Model)
|
||||||||
|---|---|---|---|---|---|---|---|---|
| SEBAS Females | MIDUS Females | |||||||
|
|
|
|||||||
| PD (P) | CV/metabolic (P) | HPA/SNS (P) | INF (NB) | PD (NB) | CV/metabolic (NB) | HPA/SNS (P) | INF (ZINB) | |
| Education Model (includes age, age-squared, race/ethnicity, education a) | ||||||||
| (Low education omitted) | ||||||||
| Medium education | 1.01 | 0.99 | 1.13 | 0.98 | 0.97 | 0.97 | 1.06 | 0.96 |
| High education | 0.84* | 0.83 | 0.94 | 0.72* | 0.83** | 0.86* | 0.92 | 0.76** |
| p-value medium vs. high education | 0.002 | 0.032 | 0.136 | 0.032 | 0.009 | 0.089 | 0.174 | 0.019 |
| χ2 (2 df) | 9.76** | 5.06 | 2.76 | 5.15 | 11.33** | 4.96 | 1.88 | 9.24** |
| Income Models (includes age, age-squared, race/ethnicity, income quartiles b) | ||||||||
| (Q1 omitted) | ||||||||
| Q2 | 0.97 | 1.01 | 1.05 | 0.90 | 0.89 | 0.91 | 1.01 | 0.80* |
| Q3 | 0.96 | 0.95 | 1.06 | 0.93 | 0.84* | 0.84* | 1.01 | 0.79* |
| Q4 | 0.78** | 0.71** | 1.10 | 0.71 | 0.75** | 0.74** | 0.88 | 0.73** |
| p-value Q2 vs. Q3 | 0.872 | 0.500 | 0.921 | 0.804 | 0.450 | 0.360 | 0.978 | 0.852 |
| p-value Q3 vs. Q4 | 0.003 | 0.004 | 0.767 | 0.105 | 0.126 | 0.211 | 0.319 | 0.556 |
| χ2 (3 df) | 12.60** | 13.17** | 0.45 | 3.99 | 18.22** | 12.05** | 1.41 | 9.13* |
| Ladder Score Models (includes age, age-squared, race/ethnicity, ladder score c) | ||||||||
| (Low ladder score omitted) | ||||||||
| Middle ladder score | 0.99 | 1.00 | 1.05 | 0.95 | 0.92 | 0.94 | 0.88 | 0.96 |
| High ladder score | 0.96 | 0.97 | 1.02 | 0.86 | 0.97 | 1.06 | 0.91 | 0.92 |
| p-value for middle vs. high | 0.567 | 0.747 | 0.832 | 0.495 | 0.457 | 0.163 | 0.727 | 0.707 |
| χ2 (2 df) | 0.62 | 0.14 | 0.20 | 1.11 | 1.74 | 1.96 | 1.63 | 0.78 |
|
| ||||||||
| N | 450 | 554 | ||||||
Note. SEBAS = Social Environment and Biomarkers of Aging Study; MIDUS = Midlife in the United States; PD = physiological dysregulation; NB = negative binomial regression model; CV/metabolic = cardiovascular and metabolic function score; P = Poisson regression model; HPA/SNS = hypothalamic–pituitary–adrenal axis and sympathetic nervous system; INF = inflammation score; ZINB = zero-inflated negative binomial.
SEBAS education categories: for males, low= 0-5 years, medium=6 years, and high= 7+ years; for females, low= 0 years, medium=1-6 years, and high=7+ years. MIDUS education categories are: high school or less, some college/2 yr degree, and 4-5 yr college degree or more.
Income quartiles SEBAS: Q1=NT$0 - NT$144,000; Q2=NT$145,500-NT$270,000; Q3=NT$271,500-NT$605,000; and Q4=NT$620,000-NT$860,000. Income quartiles MIDUS: Q1=$0-$39,000; Q2=$39,024 - $62,500; Q3 = $62,750-$101,250; and Q4 = $101,500-$400,000. Note that 1 NT$ = 0.33 US$.
SEBAS ladder score categories: low=rungs 0-4; medium=rung 5; high=rungs 6-10. MIDUS ladder score categories: low=rungs 0-6; medium=rung 7; high=rungs 8-10.
p < 0.05;
p<0.01
Fewer significant relationships between SES and biological risk emerged among women (Table 4). Among both Taiwanese and US females, the overall physiological dysregulation score was significantly and negatively related (e.g. higher SES, lower risk) to education (χ2=9.76, 2 df, p<0.01 and χ2=11.33, 2 df, p<0.01, respectively) and income (χ2=12.60, 3 df, p<0.01 and χ2=18.22, 3 df, p<0.01, respectively), but was not related to the ladder score in either population. CV/metabolic risk was significantly related to only income in both populations (χ2=13.17, 3 df, p<0.01 and χ2=12.05, 3 df, p<0.01), with higher incomes associated with lower risk relative to lower income groups. HPA/SNS function was not related to SES among females. Inflammation was not significantly associated with any of the SES measures among Taiwanese females but the chi-square tests indicated that it was significantly and negatively related to education (χ2=9.24, 2 df, p<0.01) and income (χ2=9.13, 3 df, p<0.05) among US females.
Figure 1 shows SES differentials (difference between the low and high groups for a given SES variable) in predicted marginal means of the biomarker summary scores that are significantly associated with SES in the regression models. Where an SES measure is associated with biological risk in both countries, the magnitudes of the SES differentials appear to be similar, with one exception: the education differential for the PD score among men is nearly double in the US compared with Taiwan (the low-high education gap in PD score is 1.7 points in the US compared with 0.9 points in Taiwan). For the other significant associations, the SES-differentials are either equal or differ by only 0.1 or 0.2 points. There are, however, five instances in which an SES-measure is significantly associated with biological risk in one country but not the other. In two of these cases, the differences are significant in Taiwan (the ladder gap in the PD score for males and the income gap in HPA/SNS scores among males) and in three cases, the differentials are significant in the US population (education gap in CV/metabolic risk among males and education and income gaps in inflammation among females).
Figure 1.
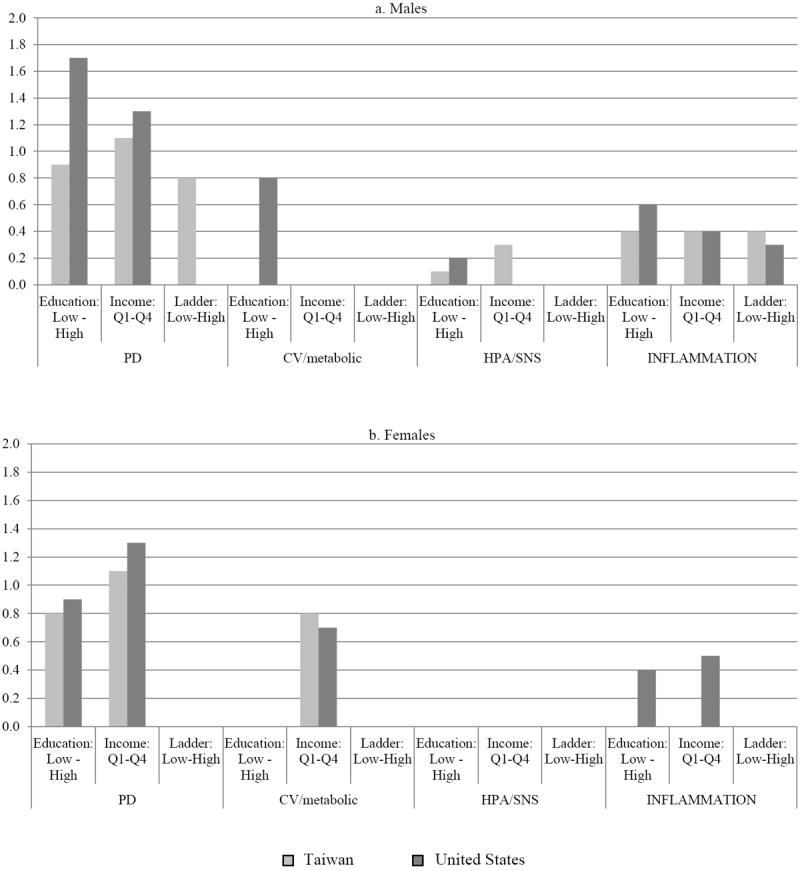
Predicted Marginal Means of Biomarker Summary Scores Significantly Associated with SES in Regression Models: Differentials between Low and High Categories of SES
DISCUSSION
In analyses presented here, we document and compare SES differentials in biological risk among middle-aged and older adults in Taiwan and the United States using a wide array of biological indicators and several measures of SES. As expected, we find significant negative SES-biological risk relationships across various physiological systems, although, as concluded by other studies (Goldman et al., 2011; Rosero-Bixby & Dow, 2009; Dowd & Goldman, 2006), results vary by measure of SES, biological risk, sex and country.
We also hypothesized that the SES differentials in biological risk would be more widespread and larger in the US than in Taiwan. However, we find that SES differentials are no more prevalent in one country than the other. Of 24 possible SES-biological summary score relationships, 12 are significant in the US compared with 11 in Taiwan. Furthermore, with the exception of the education differential in the overall PD score among men, which is nearly twice as large in the US, the magnitude of other SES differentials appear similar across countries. These results are inconsistent with the general consensus in the literature that differentials in health by education and income are wider in the US than other, primarily European, countries where inequality is lower, social safety nets are stronger, and health care more comprehensive than in the US (Avendano et al., 2009; Avendano et al., 2011; Banks et al., 2006; National Research Council, 2011; National Research Council & Institute of Medicine, 2013).
Although we can only speculate as to why the SES differences in biological risk are more similar in the two countries than anticipated, contextual factors specific to Taiwan could be contributing to the unexpected findings. For instance, Taiwan’s recent history of rapid social and economic development may influence current SES-biological risk relationships. Lifestyle and nutrition changes often accompany social and economic development. A model of such changes (Popkin & Gordon-Larson, 2004) suggests that, initially, diets become less healthy and lifestyles more sedentary. As development continues, nutrition becomes healthier and lifestyles more active (Popkin & Gordon-Larson, 2004). The timing of these transitions within a country can differ across socioeconomic status groups, with higher SES groups tending to experience each transition first (Kim, Symons, & Popkin, 2004). Taiwan’s social and economic transition from a highly agricultural to a primarily industrial and service oriented economy has occurred within the last 50-60 yars2. As a result, the nutrition and lifestyle transition could be at the point where higher SES groups have adopted healthier ways of life to a greater extent than lower SES groups, resulting in a closer relationship between SES and biological risk than might be found during other stages of the transition.
Also, the potential benefits of a universal health care system for SES-differentials in health might be attenuated by SES differences in the use of services. Although health care utilization rates are high among older Taiwanese adults and rates have increased since the implementation of the program in 1995 (Ofstedal & Natividad, 2002), there is also some evidence that utilization differs by socioeconomic status with more highly educated individuals being more likely to use services, particularly preventative services, than individuals with less education (Ofstedal & Natividad, 2002). Data also suggest that, although not widespread, unmet health care needs (e.g. having a health problem but not seeking treatment due to cost, transportation, doctor too far, lack of personal assistance, etc.) vary by SES: the probability of reporting unmet need is higher among those with lower levels of education and income compared with their higher SES counterparts (Ofstedal & Natividad, 2002). Further research is needed to assess whether contextual factors such as those discussed here influence SES differentials in biological risk.
Several additional observations regarding results of these analyses are noteworthy. First, results inform what is the appropriate outcome to examine when comparing population health across countries, suggesting that both cumulative biological risk (PD score) and physiological system subscores may be important. We find that the PD score is consistently associated with education and income across all four population subgroups. However, SES differences in cumulative biologic risk do not offer deeper understanding of the mechanistic pathways linking SES to disease and death. By examining system subscores, we learn that SES differences in overall biological risk are most influenced by inflammation, the subscore most consistently associated with SES, particularly among males. To address SES disparities in morbidity and mortality it may be particularly fruitful to focus on etiologies linked with inflammatory processes.
Second, we find that biological risk varies in both countries by education and income, particularly the PD and inflammation scores, but varies much less by the ladder score. These biological indices, therefore, appear to be influenced more by the costs or benefits of objective or absolute SES position rather than the potential effects of perceived relative status. Although these results run counter to studies that report equally strong, or stronger, relationships between self-reported health measures and subjective social status vs. objective status measures (Chen et al., 2012; Hu et al. 2005; Singh-Manoux et al., 2005), they are consistent with studies that find that subjective social status is less strongly associated with biological indicators than with self-reports of health (Demakakos et al., 2008; Adler et al 2008). Self-reported health measures, such as global health, depression, or physical functioning, may be more closely related to subjective social status than biological markers because self-reported measures and subjective social status may share a common underlying cognitive process (Demakakos et al., 2008) or because self-reported measures tend to be broader measures of health that encompass a wider range of pathways through which perceived status may matter (Adler et al., 2008). Additional research is needed to test such hypotheses.
Finally, clear gender differences in the SES-biological risk relationship emerge in the Taiwan population: SES disparities in risk are more prevalent among men than women. The relatively weaker association between SES and biological risk among Taiwanese women compared with Taiwanese men is not surprising given the limited opportunities for education and labor force participation experienced by the cohorts of women in this study (Hermalin, Liu, & Freedman 1994). The link between SES and risk among Taiwanese women might also be attenuated by the fact that their lives were largely organized around the family in which status depended on men in the family (Baker 1979). It will be interesting to see whether these gender differences in SES-biological risk relationships change with the aging of future cohorts of Taiwanese in which women will have more education, greater labor force participation, and spend more time outside the family.
Our study has several limitations. First, although we included a large number of biomarkers in our analyses, the assays used to measure the biomarkers differed across the two studies and were performed at different laboratories, which constrained our ability to make comparisons of absolute levels across countries and could account for some of the cross-country differences in results (Goldman et al., 2011). Second, we focused on cohorts of middle-aged and older adults and cannot generalize beyond these populations. This consideration is particularly important for populations such as Taiwan that have experienced rapid advances in health and social and economic development. Such improvements are likely to have resulted in significant generational differences in factors such as early-childhood exposures and educational attainment that can affect health later in life (Finch & Crimmins, 2004; Masters, Hummer, & Powers, 2012). Finally, our analyses of educational disparities of biological risk in the US are limited somewhat by the highly educated composition of the MIDUS II biological study sample (a low proportion of respondents had a high school degree or less; see Table 1). With less variation in education, these gradients in biological risk in the US may be underestimated, particularly in light of evidence that suggests that those with the worst health are individuals with less than a high school education (Braveman et al., 2010).
Research has shown that the US has worse health than many industrialized countries. The disadvantage occurs at every level of socioeconomic status, but is widest at the lowest levels of SES. This larger gap between the haves and the have-nots could be one factor contributing to the US’s overall health disadvantage. Because SES primarily works through biological risk factors to affect health, we compared SES differentials in biological risk in the US to differentials in Taiwan, a country that differs from the US in terms of health care coverage, strength of the social safety net, and social inequality. Unlike other comparisons that have been made, SES disparities in risk generally were neither more prevalent nor wider in the US than in Taiwan, suggesting that other historical, social, economic and cultural aspects of Taiwanese society are likely shaping the SES-biological risk relationship in that country. It will be interesting to follow the trends in SES disparities in health and biological risk in Taiwan as the socio-demographic and economic characteristics of future cohorts of older adults change and in the US as the provisions of the Affordable Care Act are implemented.
Supplementary Material
Acknowledgments
We acknowledge the hardwork and dedication of the staff at the Center for Population and Health Survey Research (BHP), who were instrumental in the design and implementation of the Social Environment and Biomarkers of Aging Study (SEBAS) and supervised all aspects of the fieldwork and data processing.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by and the SEBAS was funded by the National Institute on Aging (Grants R01AG16790 and R01AG16661). The Health Promotion Administration (HPA), Department of Health, Taiwan provided additional financial support for SEBAS 2000. This work was further supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Grant R24HD047879). Funding for the Taiwan Longitudinal Study of Aging (TLSA) came from the Taiwan Department of Health, the Taiwan National Health Research Institute (Grant DD01-86IX-GR601S) and the Taiwan Provincial Government. The MIDUS I study (Midlife in the United States) was supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development. The MIDUS II research was supported by a grant from the National Institute on Aging (P01-AG020166) to conduct a longitudinal follow-up of the MIDUS I investigation. The research was further supported by the following grants: M01-RR023942 (Georgetown), M01-RR00865 (University of California Los Angeles) from the General Clinical Research Centers Program; and National Center for Advancing Translational Sciences (NCATS), National Insitute of Health (UL1TR000427).
Footnotes
For example, compared with the US, Taiwan has a lower Gini coefficient (0.31 vs. 0.38; LIS Cross-National Data Center in Luxembourg, 2012), a lower percentage of the population living below the poverty line (1.2% vs. 15.1%; Central Intelligence Agency 2012a; Central Intelligence Agency, 2012b), a lower relative poverty (60% of median income) rate (15.8 vs. 24.4; LIS Cross-National Data Center in Luxembourg 2012), and a lower ratio of disposable household income at the 90th vs. 10th percentile (4.0 vs. 5.8; LIS Cross-National Data Center in Luxembourg 2012).
Between 1952 and 2011, the percent of the labor force in the agricultural sector declined from about 56% to about 5% (Hermalin, Liu, Freedman 1994; DGBAS 2013).
Contributor Information
Jennifer C. Cornman, Consultant, 113 Chapin Place, Granville, OH 43023, jencornman@gmail.com, Phone/Fax: 740.587.7956.
Dana A. Glei, Center for Population Health, Georgetown University, Washington, D.C. USA.
Noreen Goldman, Office of Population Research, Princeton University, Princeton, NJ, USA.
Carol D. Ryff, Institute on Aging, University of Wisconsin, Madison, WI, USA.
Maxine Weinstein, Center for Population Health, Georgetown University, Washington, D.C. USA.
References
- Adler N, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: preliminary data in healthy white women. Health Psychology. 2000;19:586–92. doi: 10.1037//0278-6133.19.6.586. [DOI] [PubMed] [Google Scholar]
- Adler NE, Ostrove JM. Socioeconomic status and health: what we know and what we don’t. Annals New York Academy of Sciences. 1999;896:3–15. doi: 10.1111/j.1749-6632.1999.tb08101.x. [DOI] [PubMed] [Google Scholar]
- Adler N, Singh-Manoux A, Schwartz J, Stewart J, Matthews K, Marmot MG. Social status and health: a comparison of British civil servants in Whitehall-II with European- and African-Americans in CARDIA. Social Science & Medicine. 2008;66:1034–1045. doi: 10.1016/j.socscimed.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Adler NE, Stewart J with the Psychosocial Working Group. San Francisco, CA: John D. and Catherine T. MacArthur Foundation; 2009. [March; 30 March 2009]. The MacArthur scale of subjective social status. [homepage opn the Internet] updated 2007 March; Available from: http://www.macses.ucsf.edu/research/psychosocial/subjective.php. [Google Scholar]
- Avendano M, Glymour MM, Banks J, Mackenbach JP. Health Disadvantage in US adults aged 50 to 74 years: A Comparison of the Health of Rich and Poor Americans with that of Europeans. American Journal of Public Health. 2009;99:540–548. doi: 10.2105/AJPH.2008.139469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avendano M, Kok R, Glymour M, Berkman L, Kawachi I, Kunst A, Mackenbach J. Do Americans have higher mortality than Europeans at all levels of the education distribution?: A comparison of the United States and 14 European countries. In: Crimmins EM, Preston SH, Cohen B, editors. International differences in mortality at older ages: Dimensions and sources. Washington, DC: The National Academies Press; 2011. [July 24, 2013]. pp. 313–332. at http://www.nap.edu/openbook.php?record_id=12945. [Google Scholar]
- Baker HDR. Chinese Family and Kinship. New York: Columbia University Press; 1979. [Google Scholar]
- Banks J, Marmot M, Oldfield Z, Smith JP. Disease and disadvantage in the United States and in England. Journal of the American Medical Association. 2006;295:2037–2045. doi: 10.1001/jama.295.17.2037. [DOI] [PubMed] [Google Scholar]
- Banks J, Smith JP. International Comparisons in health economics: Evidence from aging studies. Santa Monica, CA: RAND Corporation; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braveman PA, Cubbin C, Egerter S, Williams DR, Pamuk E. Socioeconomic disparities in health in the United States: What the patterns tell us. American Journal of Public Health. 2010;100:S186–S196. doi: 10.2105/AJPH.2009.166082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, Posner S. Socioeconomic status in health research: One size does not fit all. Journal of the American Medical Association. 2005;294:2879–2888. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- Central Intelligence Agency. The 2012 World Factbook page on Taiwan, Section: Economy. [May 25, 2012];World Factbook. 2012a [Online database]. Available from: https://www.cia.gov/library/publications/the-world-factbook/geos/tw.html.
- Central Intelligence Agency. The 2012 World Factbook page on the United States, Section: Economy. [May 25, 2012];World Factbook. 2012b [Online database]. Available from: https://www.cia.gov/library/publications/the-world-factbook/geos/us.html>.
- Chang MC, Glei DA, Goldman N, Weinstein M. The Taiwan biomarker project. In: Weinstein M, Vaupel JW, Wachter KW, editors. Biosocial surveys. Committee on Advances in Collecting and Utilizing Biological Indicators and Genetic Information in Social Science Surveys, Committee on Population, Division of Behavioral and Social Sciences and Education, The National Academies Press; Washington, DC: 2007. pp. 3-1–3-16. [Google Scholar]
- Chang M, Lin H, Chuang Y, Goldman N, Peterson CE, Glei DA, Weinstein M, Hurng B, Lin Y, Lin S, Liu I, Liu H, Lin S, Wu C, Hsiao M, Wu S. Social Environment and Biomarkers of Aging Study (SEBAS) in Taiwan, 2000 and 2006: Main documentation for SEBAS longitudinal public use data (released 2012). ICPSR03792-v5. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor], 2012-01-06; 2012. [Google Scholar]
- Chen B, Covinsky KE, Stijacic Cenzer I, Adler N, Williams BA. Subjective social status and functional decline in older adults. Journal of General Internal Medicine. 2012;27:693–9. doi: 10.1007/s11606-011-1963-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornman JC. Unpublished doctoral dissertation. University of Michigan; Ann Arbor, MI: 1999. Understanding the ties that bind: intergenerational value agreement in Taiwan. [Google Scholar]
- Crimmins EM, Garcia K, Kim JK. Are international differences in health similar to international differences in life expectancy? . In: Crimmins EM, Preston SH, Cohen B, editors. International differences in mortality at older ages: Dimensions and sources. Panel on Understanding Divergent Trends in Longevity in High-Income Countries, Committee on Population, Division of Behavioral and Social Sciences and Education, The National Academies Press; Washington, DC: 2010. [Google Scholar]
- Crimmins EM, Kim JK, Vasunilashorn S. Biodemography: New approaches to understanding trends and differences in population health and mortality. Demography. 2010;47:S41–S64. doi: 10.1353/dem.2010.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins EM, Seeman TE. Integrating biology into the study of health disparities. Population Development Review. 2004;30:89–107. [Google Scholar]
- Demakakos P, Nazroo J, Breeze E, Marmot M. Socioeconomic status and health: the role of subjective social status. Social Science & Medicine. 2008;67:330–340. doi: 10.1016/j.socscimed.2008.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Directorate-General of Budgets, Accounts, and Statistics (DGBAS) [December 12, 2013];Table 5 Employed Persons by Industry. 2013 at http://eng.stat.gov.tw/ct.asp?xItem=12683&ctNode=1609&mp=5.
- Dowd JB, Goldman N. Do biomarkers of stress mediate the relation between socioeconomic status and health? Journal of Epidemiology and Community Health. 2006;60:633–639. doi: 10.1136/jech.2005.040816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eibner C, Evans WN. Relative deprivation, poor health habits, and mortality. The Journal of Human Resources. 2005;40:591–620. [Google Scholar]
- Elo IT. Social class differentials in health and mortality: patterns and explanations in comparative perspective. Annual Review of Sociology. 2009;35:553–572. [Google Scholar]
- Finch CE, Crimmins EM. Inflammatory exposure and historical changes in human life-spans. Science. 2004;305:1736–1739. doi: 10.1126/science.1092556. [DOI] [PubMed] [Google Scholar]
- Franzini L, Frenandez-Esquer ME. The association of subjective social status and health in low-income Mexican-origin individuals in Texas. Social Science & Medicine. 2006;63:788–804. doi: 10.1016/j.socscimed.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Glei DA, Goldman N, Lin YH, Weinstein M. Relaxation practice and physiological regulation in a national sample older Taiwanese. Journal of Alternative & Complementary Medicine. 2012;18:653–661. doi: 10.1089/acm.2010.0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman N, Cornman JC, Chang MC. Measuring subjective social status: a case study of older Taiwanese. Journal of Cross Cultural Gerontology. 2006;21:71–89. doi: 10.1007/s10823-006-9020-4. [DOI] [PubMed] [Google Scholar]
- Goldman N, Dowd JB. Considering the inclusion of metabolic and cardiovascular markers in the Panel Study of Income Dynamics. Biodemography and Social Biology. 2009;55:140–158. doi: 10.1080/19485560903382437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman N, Turra CM, Rosero-Bixby L, Weir D, Crimmins E. Do biological measures mediate the relationship between education and health: a comparative study. Social Science & Medicine. 2011;72:307–315. doi: 10.1016/j.socscimed.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenwald TL, Seeman TE, Ryff CD, Karlamangla AS, Singer BH. Combinations of biomarkers predictive of later life mortality. [July 24, 2013];Proceedings of the National Academy of Sciences. 2006 103(38):14158–16163. doi: 10.1073/pnas.0606215103. at http://www.pnas.org/content/103/38/14158.full.pdf+html?sid=e9750373-263b-4f31-8518-3d3b09f05b31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermalin A, Liu PKC, Freedman D. The social and economic transformation of Taiwan. In: Thornton A, Lin H-S, editors. Social Change and the Family in Taiwan. Chicago: University of Chicago Press; 1994. pp. 49–87. [Google Scholar]
- Hermalin AI, Ofstedal MB, Sun C, Llu I-W. Nativity differentials in older age mortality in Taiwan: Do they exist and why? Ren Kou Xue Kan. 2009;39:1–58. [PMC free article] [PubMed] [Google Scholar]
- Hu P, Adler NE, Goldman N, Weinstein M, Seeman TE. Relationship between subjective social status and measures of health in older Taiwanese persons. Journal of the American Geriatrics Society. 2005;53:483–488. doi: 10.1111/j.1532-5415.2005.53169.x. [DOI] [PubMed] [Google Scholar]
- Huang C-C, Ku Y-W. Effectiveness of social welfare programs in East Asia: A case study of Taiwan. Social Policy & Administration. 2011;45:733–751. [Google Scholar]
- Jouven X, Empana J-P, Schwartz PJ, Desnos M, Courbon D, Ducimetiere P. Heart-Rate profile during exercise as a predictor of sudden death. The New England Journal of Medicine. 2005;352:1951–1958. doi: 10.1056/NEJMoa043012. [DOI] [PubMed] [Google Scholar]
- Kim S, Symons M, Popkin BM. Contrasting socioeconomic profiles related to healthier lifestyles in China and the United States. American Journal of Epidemiology. 2004;159:184–191. doi: 10.1093/aje/kwh006. [DOI] [PubMed] [Google Scholar]
- Kondo N, Kawachi I, Subramanian SV, Takeda Y, Yamagata Z. Do social comparisons explain the association between income inequality and health?: Relative deprivation and perceived health among male and female Japanese individuals. Social Science and Medicine. 2008;67:982–987. doi: 10.1016/j.socscimed.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIS Cross-National Data Center in Luxembourg. Luxembourg Income Study Inequality and Poverty Key Figures; c2010. [May 25, 2012]; Available from http://www.lisdatacenter.org/lis-ikf-webapp/app/search-ikf-figures?s=inequlaity+measures#.
- Love GD, Seeman TE, Weinstein M, Ryff C. Bioindicators in the MIDUS national study: protocol, measures, sample, and comparative context. Journal of Aging and Health. 2010;22:1059–1080. doi: 10.1177/0898264310374355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchenko Y, Royston P. What is the relation between the official multiple-imputation command, mi, and the user-written ice and mim commands? StataCorps; College Station, TX: 2009. [January 21, 2010]. Available at http://www.stata.com/support/faqs/stat/mi_ice.html. [Google Scholar]
- Masters RK, Hummer R, Powers DA. Educational differences in U.S. adult mortality: a cohort perspective. American Sociological Review. 2012;77:584–572. doi: 10.1177/0003122412451019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Seeman TE. Protective and damaging effects of mediators of stress. In: Adler NE, Marmot M, McEwen BS, editors. Socioeconomic status and health in industrial nations: Social, psychological and biological pathways. New York: Academic of Sciences; 1999. pp. 30–47. [Google Scholar]
- National Cholesterol Education Program, National Heart, Lung and Blood Institute. Bethesda, MD: National Institutes of Health; 2001. Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) Report No.: NIH publication no. 01-3670. URL: http://www.nhlbi.nih.gov/guidelines/cholesterol/atp3full.pdf. [PubMed] [Google Scholar]
- National Research Council. Explaining Divergent Levels of Longevity in High-Income Countries. Washington, DC: The National Academies Press; 2011. [October 1, 2013]. at http://www.nap.edu/catalog.php?record_id=13089. [PubMed] [Google Scholar]
- National Research Council and Institute of Medicine. U S Health in International Perspective: Shorter Lives, Poorer Health. Washington, DC: The National Academies Press; 2013. [PubMed] [Google Scholar]
- Ofstedal MB, Chan A, Chayovan N, Chuang AP, Mehta K, Hermalin AI. Policies and programs in place and under development. In: Hermalin AI, editor. The Well-Being of the Elderly in Asia: A Four-Country Comparative Study. Ann Arbor: University of Michigan Press; 2002. pp. 65–99. [Google Scholar]
- Ofstedal MB, Natividad JN. Patterns of health care utilization. In: Hermalin AI, editor. The Well-Being of the Elderly in Asia: A Four-Country Comparative Study. Ann Arbor: University of Michigan Press; 2002. pp. 413–459. [Google Scholar]
- Popkin BM, Gordon-Larson P. The nutrition transition: worldwide obesity dynamics and their determinants. International Journal of Obesity. 2004;28:S2–S9. doi: 10.1038/sj.ijo.0802804. [DOI] [PubMed] [Google Scholar]
- Radler BT, Ryff CD. Who participates? accounting for longitudinal retention in the MIDUS national study of health and well-being. Journal of Aging and Health. 2010;22:307–331. doi: 10.1177/0898264309358617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosero-Bixby L, Dow WH. Surprising SES gradients in mortality, health and biomarkers in a Latin American population of adults. J Gerontol B Psychol Sci Soc Sci. 2009;64B:105–117. doi: 10.1093/geronb/gbn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryff C, Almeida DM, Ayanian JS, Carr DS, Cleary PD, Coe C, Williams D, et al. Midlife Development in the United States (MIDUS II), 2004e2006. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [Distributor]; 2007. [Computer file]. No ICPSR04652-v1. [Google Scholar]
- Ryff CD, Seeman T, Weinstein M. National Survey of Midlife Development in the United States (MIDUS II): Biomarker Project, 2004-2009. ICPSR29282-v4. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor], 2013-05-02; 2013. [DOI] [Google Scholar]
- Schooling CM, Jiang CQ, Lam TH, Zhang WS, Cheng KK, Leung GM. Life-course origins of social inequalities in metabolic risk in the population of a developing country. American Journal of Epidemiology. 2008;167:419–428. doi: 10.1093/aje/kwm329. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Crimmins E, Huang MH, Singer B, Bucur A, Gruenewald T, Berkman LF, Reuben DB. Cumulative biological risk and socio-economic differences in mortality: MacArthur Studies of Successful Aging. Social Science & Medicine. 2004;58:1985–97. doi: 10.1016/S0277-9536(03)00402-7. [DOI] [PubMed] [Google Scholar]
- Seeman T, Merkin SS, Crimmins E, Koretz B, Charette S, Karlamangla A. Education, income and ethnic differences in cumulative biological risk profiles in a national sample of US adults: NHANES III (1988–1994) Social Science & Medicine. 2008;66:72–87. doi: 10.1016/j.socscimed.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Manoux A, Adler NE, Marmot MG. Subjective social status: Its determinants and its association with measures of ill-health in the Whitehall II study. Social Science & Medicine. 2003;56:1321–33. doi: 10.1016/s0277-9536(02)00131-4. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, Marmot MG, Adler NE. Does subjective social status predict health and change in health status better than objective status? Psychosomatic Medicine. 2005;67:855–861. doi: 10.1097/01.psy.0000188434.52941.a0. [DOI] [PubMed] [Google Scholar]
- Squires DA. The U S health system in perspective: A comparison of twelve industrialized nations. New York: The Commonwealth Fund; 2011. [July 24, 2013]. at http://www.commonwealthfund.org/~/media/Files/Publications/Issue%20Brief/2011/Jul/1532_Squires_US_hlt_sys_comparison_12_nations_intl_brief_v2.pdf. [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP; 2009. [Google Scholar]
- Subramanyam M, Kawachi I, Berkman L, Subramanian SV. Relative deprivation in income and self-rated health in the United States. Social Science and Medicine. 2009;69:327–34. doi: 10.1016/j.socscimed.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Thorpe KE, Howard DH, Galactionova K. Differences in disease prevalence as a source of the US-European health care spending gap. Health Affairs. 2007;26:w678–w686. doi: 10.1377/hlthaff.26.6.w678. [DOI] [PubMed] [Google Scholar]
- Wilkinson RG, Pickett KE. Income inequality and population health: a review and explanation of the evidence. Social Science & Medicine. 2006;62:1768–1784. doi: 10.1016/j.socscimed.2005.08.036. [DOI] [PubMed] [Google Scholar]
- Wilkinson RG, Pickett KE. The problems of relative deprivation: why some societies do better than others. Social Science & Medicine. 2007;65:1965–1978. doi: 10.1016/j.socscimed.2007.05.041. [DOI] [PubMed] [Google Scholar]
- Williams DR, Sternthal M. Understanding racial/ethnic disparities in Health: Sociological contributions. Journal of Health and Social Behaviour. 2010;51:S15–S27. doi: 10.1177/0022146510383838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CE, Steptoe A. Subjective socioeconomic position, gender and cortisol responses to waking in an elderly population. Psychoneuroendocrinology. 2005;30:582–90. doi: 10.1016/j.psyneuen.2005.01.007. [DOI] [PubMed] [Google Scholar]
- World Health Organization. BMI Classification. [July 21, 2013];2013 at - http://apps.who.int/bmi/index.jsp?introPage=intro_3.html.
- Zimmet P, George K, Albertini MM, Rios MS. A New International Diabetes Federation (IDF) Worldwide Definition of the Metabolic Syndrome: the Rationale and the Results. Revista Española de Cardiología (English Version) 2005;58:1371–1375. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


