Abstract
Background
Emergence of visual and musical creativity in the setting of neurologic disease has been reported in patients with semantic variant primary progressive aphasia (svPPA), also called semantic dementia (SD). It is hypothesized that loss of left anterior frontotemporal function facilitates activity of the right posterior hemispheric structures, leading to de novo creativity observed in visual artistic representation. We describe creativity in the verbal domain, for the first time, in three patients with svPPA.
Methods
Clinical presentations are carefully described in three svPPA patients exhibiting verbal creativity, including neuropsychology, neurologic exam, and structural MRI. Voxel based morphometry (VBM) was performed to quantify brain atrophy patterns in these patients against age-matched healthy controls.
Results
All three patients displayed new-onset creative writing behavior and produced extensive original work during the course of disease. Patient A developed interest in wordplay and generated a large volume of poetry. Patient B became fascinated with rhyming and punning. Patient C wrote and published a lifestyle guidebook. An overlap of their structural MR scans showed uniform sparing in the lateral portions of the language-dominant temporal lobe (superior and middle gyri) and atrophy in the medial temporal cortex (amygdala, limbic cortex).
Conclusions
New-onset creativity in svPPA may represent a paradoxical functional facilitation. A similar drive for production is found in visually artistic and verbally creative patients. Mirroring the imaging findings in visually artistic patients, verbal preoccupation and creativity may associate with medial atrophy in the language-dominant temporal lobe but sparing of lateral dominant temporal and non-dominant posterior cortices.
Keywords: creativity, dementia, aphasia, frontotemporal dementia, semantic variant primary progressive aphasia, semantic dementia, verbal creativity, visual creativity
Introduction
Some patients with frontotemporal dementia (FTD) develop heightened artistic expression in the setting of disease (Chatterjee, 2004; Miller, Marcel, Benson, Cummings, & Mena, 1996; Miller et al., 1998). In particular, de novo productivity in visual arts and music has been observed mainly in patients with semantic variant primary progressive aphasia (svPPA), or semantic dementia (SD) (Miller, Boone, Cummings, Read, & Mishkin, 2000), a clinical subtype of FTD that involves asymmetric degeneration of the language-dominant anterior temporal brain structures. It is hypothesized that slow loss of the language-dominant anterior temporal lobe function facilitates remodeling or heightened function of the non-dominant hemisphere posterior structures (Seeley et al., 2008).
Typical features of svPPA include semantic memory impairment, such as language and object recognition deficits, and behavior abnormalities, including mental rigidity and loss of empathy (Edwards-Lee et al., 1997; Hodges, Patterson, Oxbury, & Funnell, 1992; Rosen et al., 2006; Seeley et al., 2005; Snowden et al., 2001). Hypergraphia, defined narrowly as a compulsion to produce written verbal material that results in excessive or idiosyncratic writing habits, has been described previously in patients with temporal injury or dysfunction (Imamura, Yamadori, & Tsuburaya, 1992; Waxman & Geschwind, 2005; Yamadori, Mori, Tabuchi, Judo, & Mitani, 1986). These patients write compulsively and often produce excessive lists, notes, or schedules to supplement their memory for words and events. Here, however, we have observed a subset of svPPA patients for whom the term “hypergraphia” does not fully capture the quality of their writing production, which was creative rather than simply utilitarian in nature. Though visual creativity has been well-documented in these patients, increased engagement in creative writing has never been formally reported in any variant of FTD. We hypothesized that non-dominant anterior temporal lobe degeneration could release language circuits leading to increased verbal activity despite progressive impoverishment of semantic knowledge. We hereby report three svPPA patients who exhibited verbal creativity.
Methods
Chart Review
We examined verbal creativity in 95 patients evaluated at the UCSF Memory and Aging Center between 2001 and 2013 who met the older Neary research criteria for semantic dementia, now called svPPA (Gorno-tempini et al., 2011; Neary et al., 1998). Only patients with neuroimaging and at least one full clinical evaluation were included. Patients were identified to exhibit verbal creativity if they developed heightened interest in language or word play, or spontaneously generated a significant volume of work that could be categorized as creative writing, i.e. poetry or essays, which occurred or persisted after the onset of disease. Patients whose writing primarily served a functional purpose, i.e. as memory aids, were excluded from this case series. We identified three verbally creative patients (Figure 1). All study participants provided written informed consent, and all study procedures were approved by local institutional review board.
Figure 1.

Patient writing samples.
A and B: Sample poetry composed by the first patient (Case A), taken from a series of 21 poems with a centralized theme on nature and peace. C: Composition from the second patient (Case B). The third patient published a book; no sample is displayed here to protect her identity.
Imaging
T1 weighted structural MRI scans (Siemens, Inc) were acquired at the UCSF Memory and Aging Center on all patients and controls, matched in age and scanner type. Images were acquired from a 3T scanner for Case A (2008) and C (2012) and their control cohorts (72 for Case A and 79 for Case C), and from a 1.5T scanner for Case B (2005) and his control cohort (142). The following parameters were applied for 3T scans: TR/TE/TI = 2300ms/2.98ms/900ms, slice thickness = 1.0 mm, FOV = 256 mm, matrix 256 × 230, voxel size 1.0 × 1.0 × 1.0mm, and flip angle = 9°. 1.5T scans were acquired with the following parameters: TR/TE/TI = 9ms/4ms/300ms, slice thickness =1.5 mm, FOV=256 mm, matrix 256×256, voxel size 1.0×1.5×1.0mm, and flip angle =15°.
We used SPM 5 software package for image preprocessing in Voxel-based Morphometry analysis (Wellcome Department of Cognitive Neurology, London; http://www.fil.ion.ucl.ac.uk/spm). Voxel-based Morphometry is a whole-brain semi-automated procedure that involves intensity-based tissue segmentation of gray and white matter (Ashburner & Friston, 2000; Good et al., 2001). The segmented gray matter tissue scans are then normalized to a common template in MNI space. The scans are subjected to a process called modulation to preserve the original gray matter volumes before and after spatial normalization. The segmented, normalized and modulated grey matter volumes are then subjected to smoothing by using a 12 mm smoothing Kernel.
Statistical analysis was done by implementing the general linear model to define significant grey matter regional intensities. We used MATLAB-based Voxel-based lesion-symptom mapping toolbox (Bates et al., 2003; http://www.neuroling.arizona.edu), and entered age, handedness, sex, and total intracranial volume as nuisance covariate in the statistical model (Figure 2).
Figure 2.
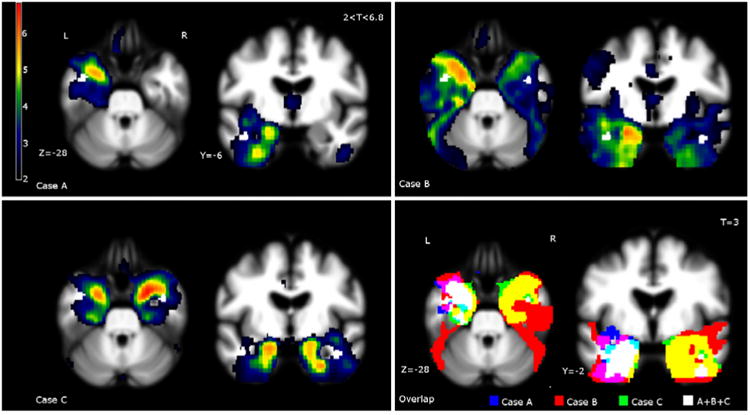
Single-subject VBM and overlap of the three cases.
Case A: Bilateral, left greater than right, anterior temporal atrophy extending to the hippocampus and orbitofrontal areas. Case B: Bilateral, left greater than right, anterior temporal lobe atrophy. Case C: Bilateral, right greater than left, atrophy in the anterior temporal lobes and amygdala. Moderate bilateral a trophy in the orbitofrontal cortex, anterior insula, and parahippocampus. Overlap: To demonstrate overlap of damage in language dominant regions, Case B has been L-R flipped because of his right-dominant language organization. All three patients had relative sparing in the dominant (shown as left) lateral superior temporal lobe, including the superior and middle gyri, and uniform atrophy in the medial temporal cortex, including the amygdala, insula, and parahippocampalgyri.
Neuropsychological Testing
A comprehensive neuropsychological exam was administered on each patient (Kramer et al., 2003). Their testing profile is shown in Table 1.
Table 1.
Patient Demographics, Functional Assessment, and Neuropsychological Performance Profiles.
| Controls N=85 | svPPA N=83 | Case A | Case B | Case C | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age | 75.1±10.0 | 63.9±7.5 | 56 | 61 | 69 |
| Years from first symptom | -- | 3.4±1.9 | 5 | 4 | 6 |
| Gender | 61.6(%Female) | 61.1 (%Female) | Female | Male | Female |
| Race | 93.0(%Caucasian) | 95.8 (%Caucasian) | Caucasian | Caucasian | Caucasian |
| Years of Education | 16.7±2.7 | 15.7±3.0 | 14 | 11 | 18 |
| Functional Assessment | |||||
| Functinoal Activities Questionnaire | 0.3±1.0 | 13±9.6 | 15 | 22 | 2 |
| Clinical Dementia Rating Box score | 0.3±0.7 | 4.9±3.9 | 4 | 5.5 | 2 |
| Clinical Dementia Rating Global | 0.1±0.2 | 0.9±0.66 | 0.5 | 1 | 0.5 |
| Tests | |||||
| MMSE (max=30) | 29.7±0.6 | 20.5±8.3 | 26 | 30 | 28 |
| Geriatric Depression Scale (max=30) | 3.3±3.1 | 9.1±7.2 | 1 | 3 | 2 |
| LANGUAGE | |||||
| Boston Naming Test (max=15) | 14.7±1.9 | 4.4±3.7 | 3 | 8 | 12 |
| Lexical Fluency (# D-words/minute) | 16.0±4.1 | 6.8±4.1 | 6 | 13 | 6 |
| Semantic Fluency (# animals/minute) | 23.8±4.6 | 7.1±5.4 | 8 | 13 | 14 |
| WRAT-4 Reading (max=70) | 65.4±3.1 | 50.1±15.6 | NA | NA | 64 |
| PPT- Picture (max=52) | 51.0±1.2 | 38.9±8.2 | 46 | NA | 50 |
| PPT-Word (max=52) | 51.6±1.0 | 37.8±10.3 | 45 | NA | NA |
| VERBAL MEMORY | |||||
| CVLT-SF Verbal Learning Trials 1-4 (max=36) | 31.3±2.4 | 13.7±7.5 | 8 | 27 | 24 |
| CVLT-SF 30″ Recall (max=9) | 8.2±0.8 | 2.4±2.4 | 3 | 8 | 6 |
| CVLT-SF 10′ Recall | 7.9±1.5 | 1.6±2.2 | 2 | 8 | 0 |
| CVLT-SF Recognition | 8.8±0.4 | 5.8±2.6 | 8 | 9 | 7 |
| VISUAL MEMORY | |||||
| Benson Figure 10′ Recall | 12.5±2.5 | 6.4±4.7 | 15 | 6 | 0 |
| VISUAL SPATIAL | |||||
| Benson Complex Figure Copy (max=17) | 15.6± 1.1 | 15.0±2.8 | 17 | 15 | 15 |
| VOSP Number Location (max=10) | 9.4±0.2 | 9.0±1.3 | 10 | 9 | 10 |
| EXECUTIVE FUNCTIONING | |||||
| Span of Digits Backward | 5.4±1.3 | 4.5±1.1 | 4 | 6 | 5 |
| Design Fluency (#/minute) | 11.5±2.9 | 7.3±4.5 | 7 | NA | 6 |
| Stroop Interference (# words/minute) | 54.8±11.3 | 32.9±15.3 | 36 | 40 | 47 |
| Calculations (max=5) | 4.8±0.6 | 4.3±1.2 | 4 | 4 | 4 |
85 controls and 83 patients with svPPA contributed valid test data and are included in the table. All test data represented as raw score. MMSE=Mini Mental Status Examination; WRAT-4 Reading = Wide Range Achievement Test 4, Reading Assessment; PPT-Picture= Pyramids and Palm Trees - Picture version; PPT-Word= Pyramids and Palm Trees - Word version; CVLT-SF= California Verbal Learning Test - Short Form; VOSP= Visual Object and Space Perception Battery. NA= data not available. Value displayed after ± indicates standard deviation.
Case A
A 56-year-old right-handed woman presented with five years of progressive language and behavioral changes. At age 51, she exhibited word substitutions – saying “medicine” for “poison” and “strawberry” for “cherries” – and had difficulty recognizing the meaning of words. At 53 she became more self-centered and was overly familiar with strangers. She acquired new spiritual ideas and talked about how “nature inspired her to write beautiful things”. Previously uninterested in writing, she created poems about communication and open-mindedness, spending hours in front of the computer re-arranging and reading aloud the words in her poems. At 56, she compiled a large volume of poems with titles such as “Clouds bring peace” and “Rainbow is giving love” (Figure 1). At the end of the year, her interest in writing suddenly disappeared.
On examination, she was euphoric and loquacious with profound anomia and deficits in semantic knowledge. Visual memory and visuospatial function were normal while verbal memory was impaired. Frontal/executive function was not significantly impaired. MRI showed bilateral, left greater than right, anterior temporal atrophy extending to hippocampal and orbitofrontal regions.
Case B
A 61-year-old ambidextrous man presented with four years of language and personality changes, including prosopagnosia. At age 57 he was unable to recognize friends and at 59, he failed to recognize his daughter. Previously altruistic, he became selfish and insisted that “after many years” it was “his turn” to get his way. At 60, he could no longer order meals in restaurants due to trouble reading the menu. He exhibited idiosyncratic word usage, and semantic paraphasic errors substituting “that boy” for “son”, and “knob” for “ankle”. A new fascination with words manifested as pressured speech, rhyming, frequent punning, and interest in word games and puzzles. He created montages with images and his own poems, highlighting play on words, rhymes, and puns (Figure 1). He developed increased religious fervor.
On examination, he spoke in rhymes and puns but made semantic and phonemic paraphasic errors. There was a marked anomia. Frontal/executive function and visual memory were impaired, but short-term verbal memory, repetition, and comprehension were intact. Based on clinical history and specific language features, he was found to meet criteria for svPPA. MRI revealed left greater than right anterior temporal lobe atrophy. A clinical read of his functional MEG study suggested he displayed reversed (right-sided) dominance for language (personal communication, March 1, 2013).
Case C
A 69-year-old right-handed woman came in with six years of language and personality changes. Previously a teacher, school district administrator, and successful entrepreneur, she had never exhibited any special interest in writing. Then at 61, she began composing a 300-page lifestyle guidebook. She worked on the book for four years, spending eight hours daily writing and perfecting the material. At 63, she became overly concerned about her diet, grew excessively talkative and more dominating in conversations, and approached strangers to hug them. After publishing the book, she introduced herself as a world-famous author to strangers. From this time forward she worked on expanding family albums – adding photo captions and editing previous written descriptions with similar intensity. At 67, she exhibited difficulty remembering the names of friends and appeared more childlike. According to her husband, she has shown increased sexual interest and openly discussed her sex life with her children. She developed interests in word jumbles, solitaire, and dominos, and played on a rigid schedule.
On examination she was jovial and childlike, occasionally sticking out her tongue, winking, and giggling. She was extremely verbose and slightly tangential. She performed poorly in tasks of semantic knowledge, executive function, and famous face recognition. MRI revealed marked atrophy in the anterior temporal lobes and amygdala, right more than left. There was moderate atrophy of the orbitofrontal cortex, right anterior insula, and right parahippocampus.
Voxel-based Morphometry (VBM) Comparisons
All three patients exhibited predominantly gray matter atrophy bilaterally in the anterior and medial temporal regions, consistent with svPPA (Brambati et al., 2009). However, Case A revealed left greater than right atrophy, while Case C showed right greater than left temporal atrophy. Case B displayed predominantly behavioral symptoms typical of right hemisphere lesion, while his MRI suggested left greater than right temporal injury. The combination of his ambidexterity, clinical presentations, and Magnetoencephalography (MEG) data suggests a strong possibility of right-hemisphere language dominance in this patient (Mesulam, Winstraub, Parrish, & Gitelman, 2005).
Despite distinct disease laterality and severity, all three patients displayed 1) sparing in the language-dominant lateral temporal lobe including the superior and middle gyrii, and 2) uniform atrophy in the medial temporal cortex, amygdala, insula, and parahippocampalgyri (Figure 2).
Discussion
In svPPA patients, an intense preoccupation with visually-mediated activities has been previously detailed (Green & Patterson, 2009; Miller et al., 1998). We describe for the first time three cases, in which a similar preoccupation with language enhanced verbal creativity in the setting of cognitive deterioration. All three of the cases with verbal creativity presented with bilateral temporal atrophy, although with different levels of asymmetry in hemisphericde generation. While patients with svPPA in general exhibit a similar core pattern of predominantly temporal atrophy, we suspect a shared distinction in these patients that would account for the novel creative abilities they developed during the course of disease. VBM of their brain anatomy showed sparing of the dorsolateral temporal language regions in all three. Conversely, there was severe involvement of the medial temporal limbic structures involved in emotion regulation and reward.
New creativity in the setting of neurodegeneration suggests that focal brain dysfunction may induce a paradoxical functional facilitation, selective improvement in performance in the context of neurological insult (Kapur, 1996). Previous studies on visual creativity in FTD revealed that brain atrophy was most severe in the language-dominant temporal lobes (Miller, Marcel, Benson, Cummings, & Mena, 1996). Focal dominant hemisphere temporal atrophy with spared, if not enhanced, dorsolateral frontal and parietal circuitry appeared to increase the desire to create paintings. As in a mirror image process, increased verbal preoccupation and creativity leading to poetry (two cases) and a book (one case) in these three individuals associated with medial temporal lobe a trophy and sparing of lateral language-dominant temporal cortex.
Hypergraphia as part of the Geschwind syndrome has been reported in both temporal lobe injury and behavioral variant frontal temporal dementia (bvFTD) (Hoffman, 2008; Postiglione et al., 2008; Waxman & Geschwind, 1975). Interestingly, the spiritual focus in Case A's poetry and the theme surrounding God in Case B's writings, Case A and C's loquaciousness, and Case C's heightened sexual interest are also reminiscent of other phenomena described in the Geschwind syndrome, namely hyper-religiosity, viscous personality, and changes in sexual behavior. Findings observed in this small case series suggests that additional investigation should be done with larger samples to better characterize any structural or functional divergence contributing to creative writing behaviors.
These three patients add to the evolving literature on creativity in dementia and demonstrate that verbal creativity can emerge in the setting of anterior temporal lobe degeneration, despite the concomitant semantic loss typical of this syndrome. This heightened verbal preoccupation has many parallels with the visual creativity demonstrated in patients with FTD.
Acknowledgments
We would like to thank our patients and families for their participation in and contributions to neurodegenerative disease research.
Study Funding: The study was supported by grant number AG032306 from NIH National Institute on Aging.
Contributor Information
T.Q. Wu, Email: wuqteresa@gmail.com, Department of Neurology, University of California, San Francisco, San Francisco, CA, Address: 675 Nelson Rising Lane, Suite 190, S.F., CA 94158. Tel: (415) 867-1506.
Z.A. Miller, Email: zmiller@memory.ucsf.edu, Department of Neurology, University of California, San Francisco, San Francisco, CA, Address: 675 Nelson Rising Lane, Suite 190, S.F., CA 94158. Tel: (415) 514-9320.
B. Adhimoolam, Email: badhimoolam@memory.ucsf.edu, Department of Neurology, University of California, San Francisco, San Francisco, CA, Address: 675 Nelson Rising Lane, Suite 190, S.F., CA 94158.
D.C. Zackey, Email: dzackey@memory.ucsf.edu, Department of Neurology, University of California, San Francisco, San Francisco, CA, Address: 675 Nelson Rising Lane, Suite 190, S.F., CA 94158. Tel: 415-476-2907.
B.K. Khan, Email: baber.khan@ucsf.edu, Department of Neurology, University of California, San Francisco, San Francisco, CA, Address: 675 Nelson Rising Lane, Suite 190, S.F., CA 94158.
R. Ketelle, Email: rketelle@memory.ucsf.edu, Department of Neurology, University of California, San Francisco, San Francisco, CA, Address: 675 Nelson Rising Lane, Suite 190, S.F., CA 94158. Tel: 415-514-2058.
K.P. Rankin, Email: krankin@memory.ucsf.edu, Department of Neurology, University of California, San Francisco, San Francisco, CA, Address: 675 Nelson Rising Lane, Suite 190, S.F., CA 94158. Tel: 415-509-8805.
B.L. Miller, Email: bmiller@memory.ucsf.edu, Department of Neurology, University of California, San Francisco, San Francisco, CA, Address: 675 Nelson Rising Lane, Suite 190, S.F., CA 94158. Tel: 415-476-5591.
References
- Ashburner J, Friston KJ. Voxel-based morphometry -- the methods. Neuro Image. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. doi:0.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT. Voxel-based lesion-symptom mapping. Nat Neurosci. 2003;6(5):448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Brambati SM, Rankin KP, Narvid J, Seeley WW, Dean D, Rosen HJ, et al. Gorno-Tempini ML, et al. Atrophy progression in semantic dementia with asymmetric temporal involvement: A tensor-based morphometry study. Neurobiol Aging. 2009;30:103–111. doi: 10.1016/j.neurobiolaging.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. The neuropsychology of visual artistic production. Neuropsychologia. 2004;42:1568–1583. doi: 10.1016/j.neuropsychologia.2004.03.011. Retrieved from http://www.elsevier.com/locate/neuropsychologia. [DOI] [PubMed] [Google Scholar]
- Edwards-Lee T, Miller BL, Benson DF, Cummings JL, Russell GL, Boone K, Mena I. The temporal variant of frontotemporal dementia. Brain. 1997;120:1027–1040. doi: 10.1093/brain/120.6.1027. [DOI] [PubMed] [Google Scholar]
- Green H, Patterson K. Jigsaws-A preserved ability in semantic dementia. Neuropsychologia. 2009;47:569–576. doi: 10.1016/j.neuropsychologia.2008.10.015. Retrieved from http://www.elsevier.com/locate/neuropsychologia. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuro Image. 2001;14:685–687. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Gorno-tempini MI, Hillis AE, Wintraub S, Kertez A, Mendez M, Cappa SF, et al. Grossman M, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic Dementia.Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115:1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Hoffman M. Isolated right temporal lobe stroke patients present with Geschwind Gastaut syndrome, frontal network syndrome and delusional misidentification syndromes. Behav Neurol. 2008;20(3):83–89. doi: 10.3233/BEN-2008-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura T, Yamadori A, Tsuburaya K. Hypergraphia associated with a brain tumor of the right cerebral hemisphere. J Neurol Neurosurg Psychiatry. 1992;55(1):25–7. doi: 10.1136/jnnp.55.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur N. Paradoxical functional facilitation in brain-behaviour research.A critical review. Brain. 1996;119:1775–1790. doi: 10.1093/brain/119.5.1775. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Jurik J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, Miller BL. Distinctive Neuropsychological Patterns in Frontotemporal Dementia, Semantic Dementia, and Alzheimer Disease. Cogn Behav Neurol. 2003;16:211–218. doi: 10.1097/00146965-200312000-00002. Retrieved from http://ovidsp.tx.ovid.com. [DOI] [PubMed] [Google Scholar]
- Mesulam M, Wintraub S, Parrish T, Gitelman D. Primary progressive aphasia: reversed asymmetry of atrophy and right hemisphere language dominance. Neurology. 2005;64:556–557. doi: 10.1212/01.WNL.0000150545.46351.DE. Retrieved from http://www.neurology.org. [DOI] [PubMed] [Google Scholar]
- Miller BL, Marcel P, Benson DF, Cummings JL, Mena I. Enhanced artistic creativity with temporal lobe degeneration. The Lancet. 1996;348(05):1744–1745. 65881–3. doi: 10.1016/S0140-6736. [DOI] [PubMed] [Google Scholar]
- Miller BL, Cummings J, Mishkin F, Boone K, Prince F, Ponton M, Cotman C. Emergence of artistic talent in frontotemporal dementia. Neurology. 1998;51(4):978–982. doi: 10.1212/wnl.51.4.978. Retrieved from http://ovidsp.ovid.com. [DOI] [PubMed] [Google Scholar]
- Miller BL, Boone K, Cummings JL, Read SL, Mishkin F. Functional correlates of musical and visual ability in frontotemporal dementia. Br J Psychiatry. 2000;176:458–463. doi: 10.1192/bjp.176.5.458. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Benson DF, et al. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. Retrieved from http://ovidsp.tx.ovid.com. [DOI] [PubMed] [Google Scholar]
- Postiglione A, Milan G, Pappata S, De Falco C, Lamenza F, Schiattarella V, et al. Striano S, et al. Fronto-temporal dementia presenting as Geschwind's syndrome. Neurocase. 2008;14(3):264–270. doi: 10.1080/13554790802269976. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Allison SC, Ogar JM, Amici S, Rose K, Dronkers N, et al. Gorno-Tempini ML. Behavioral features in semantic dementia vs other forms of progressive aphasias. Neurology. 2006;67:1752–1756. doi: 10.1212/01.wnl.0000247630.29222.34. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Bauer AM, Miller BL, Gorno-Tempini ML, Kramer JH, Weiner M, Rosen HJ. The natural history of temporal variant frontotemporal dementia. Neurology. 2005;64:1384–1390. doi: 10.1212/01.WNL.0000158425.46019.5C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Matthews BR, Crawford RK, Gorno-Tempni ML, Foti D, Mackenzie IR, Miller BL. Unraveling Bolero: progressive aphasia, transmodal creativity and the right posterior neocortex. Brain. 2008;131:39–49. doi: 10.1093/brain/awm270. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Bathgate D, Varma A, Blackshaw A, Gibbons ZC, Neary D. Distinct behavioral profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry. 2001;70:323–332. doi: 10.1136/jnnp.70.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SG, Geschwind N. Hypergraphia in temporal lobe epilepsy. Epilepsy & Behavior. 2005;6:282–291. doi: 10.1016/j.yebeh.2004.11.022. Retrieved from http://www.elsevier.com/locate/yebeh. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Geschwind N. The Interictal Behavior Syndrome of Temporal Lobe Epilepsy. Arch Gen Psychiatry. 1975;32:1580–1586. doi: 10.1001/archpsyc.1975.01760300118011. [DOI] [PubMed] [Google Scholar]
- Yamadori A, Mori E, Tabuchi M, Kudo Y, Mitani Y. Hypergraphia: a right hemisphere syndrome. J Neurol Neurosurg Psychiatry. 1986;49:1160–1164. doi: 10.1136/jnnp.49.10.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]


