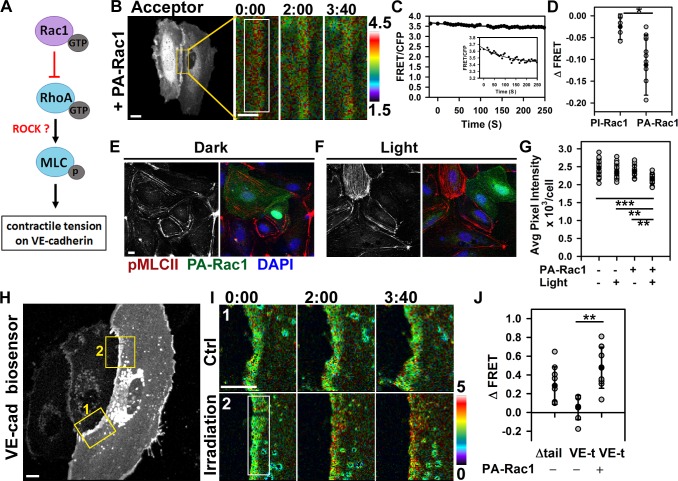
Figure 4.
Rac1 modulates actomyosin-mediated tension across VE-cadherin adhesion. (A) Model of Rac1-mediated inhibition of RhoA–ROCK signaling and mechanical force across VE-cadherin adhesion. (B and C) Cells expressing RhoA biosensor and PA-Rac1. Changes in FRET/CFP ratio after PA-Rac1 activation at time 0 within the indicated region. Ratio images were scaled from 1.5 to 4.5 and color coded as indicated on the right. Representative tracer from reproducible dataset of n = 10. The inset within C is zoomed in on the graph to show the subtle drop in FRET signal. (D) Changes in RhoA activity after photoactivation of PI-Rac1 (−0.03 ± 0.03) or PA-Rac1 (−0.11 ± 0.07); means ± SD, n = 5–10; *, P < 0.05. (E and F) Immunofluorescence staining for myosin light chain phosphorylation (pMLCII) without (dark; E) and with (light; F) Rac1 activation. (G) Quantification of p-MLCII in E and F; means ± SD, n = 11–21; **, P < 0.005; ***, P < 0.0005. (H) Cells expressing VE-cadherin (VE-cad) tension biosensor (VE-t) and PA-Rac1. Control (Ctrl; 1) and PA-Rac1 activation (irradiation; 2) zones are shown enlarged in I. (I) Spatial increase in FRET/CFP ratio was observed at the site of PA-Rac1 activation inside the rectangular region (2) but not the adjacent zone (1). Ratio images were scaled from 0 to 5. (J) Changes in FRET ratio for the tailless (tail) control and VE-t without or with PA-Rac1 activation; means ± SD, n = 7–10; **, P < 0.005. Times are given in minutes and seconds. Bars: (B [main image], E, H, and I) 10 µm; (B [insets]) 5 µm.