Abstract
Background and objectives
This study examined kidney biopsies with focal segmental glomerular fibrinoid necrosis to identify early features of pauci-immune necrotizing GN and the primary effector cells mediating initial capillary injury.
Design, setting, participants, & measurements
Seventeen consecutive kidney biopsies with focal pauci-immune necrotizing GN, obtained over a 6-year period (2007–2012), were studied. Neutrophils and CD68+, CD163+, CD3+, CD56+, and CD20+ cells were scored in paraffin sections counterstained with periodic acid–Schiff. Electron microscopy was performed in 15 of 17 biopsies and additional examples of pauci-immune necrotizing GN (n=25). Biopsies with thin basement membrane nephropathy (n=5) served as immunohistologic normal controls.
Results
Biopsies with pauci-immune necrotizing GN had a mean of 10 (range=3–25) normal-appearing glomeruli, a mean of 2 (range=1–5) glomeruli with segmental fibrinoid necrosis, and a mean of 2 (range=1–11) glomeruli with cellular crescents. CD68+ and CD163+ macrophages predominated at sites of fibrinoid necrosis in pauci-immune necrotizing GN, exceeding the quantity of neutrophils and T cells (mean scores [SD]=2.5 [0.7] and 2.2 [0.75] versus 0.6 [0.5] and 0.1 [0.3], respectively; P<0.001). B and natural killer cells were rare. Normal-appearing glomeruli in pauci-immune necrotizing GN had significantly more CD68+ and CD163+ macrophages than the controls (CD68+, 0.9 [0.3] versus 0.4 [0.3]; CD163+, 1 [0.4] versus 0.4 [0.3]; P<0.001). The quantity of other glomerular infiltrates did not differ from controls. The serum creatinine level at biopsy correlated with the glomerular CD68 and neutrophil scores (r=0.74 and r=0.71, respectively; P=0.001) but did not correlate with the extent of fibrinoid necrosis (r=0.36). Macrophages were localized at minute perforations and attenuations of the capillary basement membrane by electron microscopy.
Conclusions
Early pauci-immune necrotizing GN is characterized by a selective localization of CD163+ M2 macrophages at sites of glomerular fibrinoid necrosis and in normal-appearing glomeruli. These observations indicate that alternatively activated macrophages are positioned as potential effectors of glomerular injury in the early stages of pauci-immune necrotizing GN and may be potential targets for therapeutic intervention.
Keywords: vasculitis, macrophages, ANCA, GN
Introduction
The earliest diagnostic change in renal biopsies from patients with pauci-immune crescentic GN (CGN) is segmental fibrinoid necrosis (FN) of the glomeruli, and this is thought to be a key initial event of crescent formation (1). The mechanism of initial capillary injury is uncertain; however, ANCA with specificity for myeloperoxidase (MPO) and proteinase 3 (PR3), polymorphonuclear neutrophils (PMNs), monocytes, and macrophages are thought to contribute (2–7). A primary role for T cell–mediated immune mechanisms is inferred from studies of pauci-immune necrotizing GN (PNGN) in human ANCA-associated vasculitis (AAV) that localize T cells and macrophages in glomeruli with cellular crescents (CCs) (8–14). These prior studies of PNGN analyzed diffuse, well developed CGN, and the question of whether the T cells and macrophages serve as primary effectors or are part of a secondary response to tissue injury remains unresolved.
The goal of our study was to examine early lesions of PNGN in human renal biopsies and characterize potential effectors at sites of initial glomerular injury. We reasoned that focal and segmental glomerular injury is an earlier phase of the vasculitic process than diffuse and global glomerular injury, and we hypothesized that macrophages and PMNs are potential mediators of early vasculitic injury. Features of capillary injury that may precede exudative lesions of FN were sought at the minutest perforations of the glomerular basement membrane (GBM) by electron microscopy and in normal-appearing glomeruli from biopsies with focal PNGN. The inflammatory infiltrates were characterized by morphologic and immunohistochemical analyses.
Materials and Methods
Patients
Seventeen consecutive biopsies with focal necrotizing GN obtained over a 6-year period (2007–2012) were chosen on the basis of segmental FN affecting <50% of the glomeruli and adequate tissue for immunohistochemistry. Five patients with anti-GBM GN with focal segmental FN and eight patients with immune complex–mediated necrotizing and CGN (four patients with lupus nephritis and four patients with IgA nephropathy) with focal segmental FN were selected for comparison of infiltrating cell phenotypes. Five biopsies with thin basement membrane nephropathy were used as normal controls for immunohistochemical studies. Clinical and laboratory data were collected retrospectively from medical records. The Institutional Review Board approved this study.
Morphologic Analyses and Immunohistochemistry
Two-micrometer paraffin sections were stained using hematoxylin and eosin, periodic acid–Schiff, and Jones methenamine silver methods. We tabulated detailed glomerular histologic features from the index biopsies. The extent of tubulointerstitial inflammation, interstitial fibrosis and tubular atrophy, and arterial and arteriolar sclerosis were scored using the Banff schema for these lesions (15). Direct immunofluorescence staining for IgG, IgA, IgM, C3, C1q, κ- and λ-light chains, and fibrinogen was performed in each case. Fluorescein-labeled antibodies to IgG, IgA, IgM, κ- and λ-light chains, C3, C1q, and fibrinogen (Dako, Carpinteria, CA) were used in a standard one-step technique on 4-μm frozen sections from 16 of 17 patients and 2-μm paraffin-embedded tissue sections from one patient. The tissue samples for immunofluorescence had an average of 4.2 glomeruli (range=1–11). Immunofluorescence staining intensity was graded from 0 to 4+.
mAbs with specificity for CD68 (a general marker of macrophages, Clone PG-M1; Dako), CD163 (a marker of alternatively activated/M2-type macrophages, Clone 10D6; Novacastra, Newcastle, United Kingdom), CD3 (a marker of T cells, Clone 565; Novacastra), CD56 (a marker of natural killer cells, Clone 504; Novacastra), and CD20 (a marker of B cells, Clone L26; Dako) were used to identify inflammatory cell phenotypes using standard immunohistochemical techniques in an automated stainer (Ventana; Ventana Medical Systems, Tucson, AZ). All immunohistochemically stained sections were counterstained using periodic acid–Schiff. PMNs were identified by their segmented, densely hematoxyphilic nuclei and eosinophilic or amphophilic cytoplasm.
Scoring
To quantify glomerular inflammatory cells, the glomeruli were divided into four compartments: (1) the site of FN, (2) the endocapillary space (outside of FN), (3) the extracapillary space (outside of FN), and (4) the pericapsular compartment. For each compartment, scores of 0=0 positive cells, 1=1–4 cells, 2=5–10 cells, and 3>10 cells were applied. To perform correlation analyses, the average score for each inflammatory cell phenotype was calculated as the sum of the scores in each compartment of each glomerulus in the biopsy divided by the total number of glomeruli in the biopsy sample. This mean composite score was correlated with the serum creatinine. Interstitial inflammatory cells were scored in serial 20× fields as 0=none, 1=scattered positive cells in ≤50% of the field, 2=scattered positive cells in >50% of the field, 3=contiguous positive cells (unifocal), 4=contiguous positive cells (multifocal, <50% of the field), and 5=diffuse positivity in entire field. The scores were averaged for each case.
Electron Microscopy
Tissue was fixed in 2.5% glutaraldehyde in 0.1% Millonig’s phosphate buffer, washed, postfixed in 1% osmium tetroxide, dehydrated, and mounted in Epon resin. Ultrathin sections were stained on copper palladium grids using uranyl acetate and lead citrate. Stained sections were viewed and photographed using a Phillips CM-10 transmission electron microscope (Phillips Systems, Andover, MA).
Fifteen of 17 index patients had one or more glomeruli available for electron microscopy. An additional 25 biopsies were selected from 138 consecutive examples of PNGN received over a 5-year period (2008–2012) on the basis of having at least one glomerulus with segmental perforation of the GBM available for ultrastructural study. Multiple grids of one to four glomeruli were evaluated in each patient. Morphologic features evaluated included GBM attenuation, redundancy (defined as loss of attachment to cells or matrix) and perforation, fibrin deposition, endothelial and podocyte injury, and presence of one or more macrophages and PMNs. Inflammatory infiltrates were not further quantified.
Statistical Analyses
Data are presented as mean (SD) except where indicated. Mann–Whitney U tests were used to compare scores. Pearson correlation coefficients were determined for the scores of inflammatory cells and the serum creatinine levels at the time of biopsy. Logarithmic and square root transformations were used to normalize data as appropriate. All statistical tests were performed using Stata statistical software (Stata Corp. LP).
Results
Clinical Features
Seventeen needle core biopsies with scant staining for Igs (IgG, IgA, IgM, κ, and λ) and complement components (C3 and C1q) were classified as focal PNGN (n=16) and crescentic PNGN (n=1) by the Berden system (16) (Table 1). The study cohort had an average age of 42.2 years old; six patients were men, and 11 patients were women. Most presented with mild elevation of serum creatinine. Hematuria was evident in 17 patients, and proteinuria was present in 16 of 17 patients (0.2–2.3 g/d in 15 patients and 4 g/d in one patient). Eight patients had anti-MPO specificity, and six patients had anti-PR3 specificity. Three patients were ANCA-negative. Ten patients had extrarenal disease, and seven patients had renal limited disease. All biopsies were obtained before any medical intervention.
Table 1.
Clinical and histologic data for patients with pauci-immune necrotizing GN and controls
| PNGN (n=17) | |
| Mean age, yr (range) | 42.2 (3–74) |
| Sex | 6 men, 11 women |
| Race/ethnicity | 12 W, 3 L, 2 B |
| Proteinuria | 16 |
| Hematuria | 17 |
| Mean serum creatinine, mg/dl (range) | 1.43 (0.5–6.6) |
| Mean eGFR, ml/min per 1.73 m2 (range) | 81.3 (10.3–195.4) |
| ANCA-positive | 14 |
| Anti-MPO | 8 |
| Anti-PR3 | 6 |
| ANCA negative | 3 |
| No. of glomeruli per biopsya | 14 (5–27) |
| No. with segmental fibrinoid necrosis (range) | 2 (1–5) |
| No. with cellular crescents (range) | 2 (1–11) |
| No. with fibrous or fibrocellular crescents (range) | 0.53 (0–3) |
| No. with normal appearance (range) | 10 (3–25) |
| No. of globally sclerotic (range) | 0.6 (0–3) |
| Interstitial inflammation scoreb | 1.1±0.8 |
| Tubulitis score | 0.7±1 |
| Interstitial fibrosis score | 0.4±0.5 |
| Tubular atrophy score | 0.4±0.5 |
| Acute tubular necrosis/injury | 0 |
| Arteriosclerosis score | 0.8±0.9 |
| Arteriolosclerosis score | 0.6±0.8 |
| Controls | |
| Anti-GBM GN (n=5) | |
| No. of glomeruli per biopsya | 10 (5–14) |
| No. with segmental fibrinoid necrosis (range) | 2 (1–4) |
| Immune complex GN (n=8) | |
| SLE | 4 |
| IgAN | 4 |
| No. of glomeruli per biopsya | 24.5 (10–45) |
| No. with segmental fibrinoid necrosis (range) | 1.5 (1–4) |
| TBMN (n=5) | |
| No. of glomeruli per biopsya | 13 (8–22) |
PNGN, pauci-immune necrotizing GN; MPO, myeloperoxidase; PR3, proteinase 3; GBM, glomerular basement membrane; SLE, systemic lupus erythematosus; IgAN, IgA nephropathy; TBMN, thin basement membrane nephropathy; W, white; L, Latino; B, black.
Data given as mean (range) per biopsy.
Mean score using the Banff schema (0–3)±SD.
Light Microscopy
Segmental FN and CCs affected at least one glomerulus in all 17 biopsies (Figure 1, Table 1). Glomeruli with segmental FN had intact Bowman’s capsules. Tubulointerstitial mononuclear inflammation and vascular pathology are summarized in Table 1. One biopsy had focal necrotizing arteriolitis.
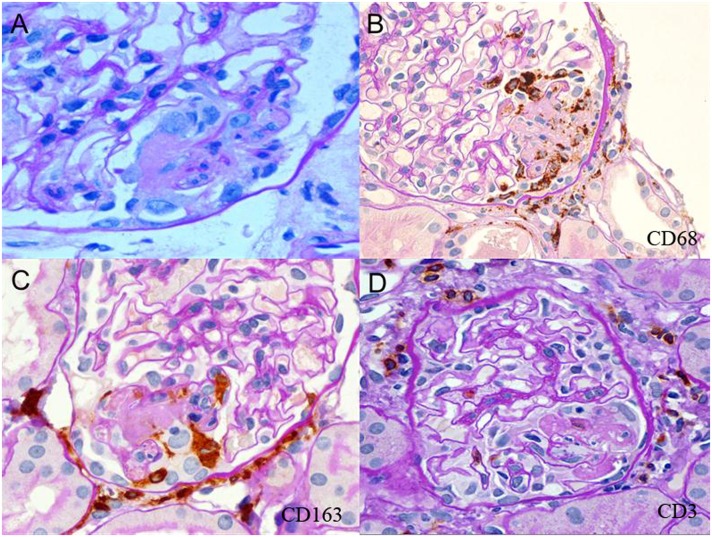
Figure 1.
Neutrophils, CD68+ and CD163+ macrophages, and rare CD3+ T cells are localized at sites of fibrinoid necrosis. (A) Segmental fibrinoid necrosis (FN) with capillary basement membrane perforation, fibrin thrombosis and exudation, polymorphonuclear neutrophils, mononuclear cells, and swollen extracapillary cells. (B) Segmental FN showing localization of CD68+ cells around and within the lesion. Pericapsular CD68+ cells are also evident. (C) Segmental FN showing localization of CD163+ cells in the lesion. Pericapsular CD163+ cells are prominent. (D) CD3+ cells were located primarily in the pericapsular region in glomeruli with segmental FN.
Immunofluorescence Microscopy
Direct immunofluorescence revealed glomerular Ig deposits in nine biopsies (IgG=0.5–1+ in six biopsies, IgA=0.5–1+ in two biopsies, and IgM=0.5–1+ in six biopsies). Granular deposits were present in the mesangium in seven biopsies and the capillary walls in two biopsies. Complement was detected in glomeruli in 10 of 16 biopsies (C3=0.5–2+ in 10 biopsies and C1q=0.5–1+ in 2 biopsies), in the mesangium in seven biopsies, and along capillary walls in four biopsies. Focal segmental staining of the glomeruli for fibrinogen was also evident in 11 biopsies.
Immunoperoxidase Studies
Infiltrating Cells at Sites of FN in PNGN.
Light microscopic GBM rupture was observed with and without fibrin deposition (Figures 1 and 2). FN had numerous CD163+CD68+ cells within and surrounding fibrinoid exudates (Figure 1, B and C). PMNs were also frequently localized at these sites (Figure 1A).
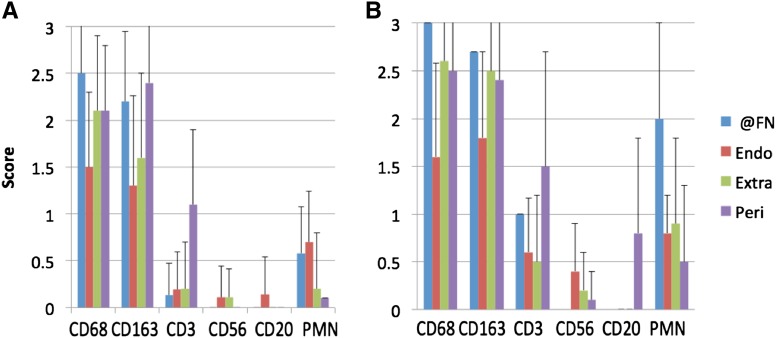
Figure 2.
Macrophages are more numerous in glomeruli with FN and cellular crescents (CCs) from biopsies with pauci-immune necrotizing GN. (A) CD68+ and CD163+ macrophages are the most abundant infiltrates in glomeruli with segmental FN, exceeding polymorphonuclear neutrophils (PMNs), T cells, B cells, and natural killer cell infiltrates (data for 28 glomeruli from 14 patients; mean+SD). (B) Macrophages, PMNs, and T infiltrates are more pronounced at sites of FN and in endocapillary, extracapillary, and pericapsular sites in glomeruli with CCs. Pericapsular B cells are also more prominent in glomeruli with CCs (data for 32 glomeruli from 15 patients; mean+SD). Endo, endocapillary; Extra, extracapillary; @FN, at the site of fibrinoid necrosis; Peri, pericapsular.
CD163+CD68+ cells exceeded the quantity of other inflammatory cell phenotypes in glomeruli with FN (n=28 glomeruli with lesions of interest studied by immunoperoxidase) (Figure 2). At sites of glomerular FN, CD68+ and CD163+ cells exceeded CD3+ cells and PMNs (average score [SD] for CD68+ cells=2.5 [0.7] and CD163+ cells=2.2 [0.75] versus 0.1 [0.34] for CD3+ cells and 0.6 [0.5] for PMNs; P<0.001 for each). The differences in scores of CD163+ and CD68+ cells were not significant (P=0.18). CD56+ and CD20+ cells were not identified at sites of FN. The endocapillary cell population in glomeruli with segmental FN consisted predominantly of CD68+ (score=1.5 [0.8]) and CD163+ cells (1.3 [0.96]), each of which exceeded CD3+ cells (0.2 [0.4]) and PMNs (0.7 [0.54]; P<0.001 for each comparison). PMNs exceeded CD3+ cells in the endocapillary compartment (P=0.007). Extracapillary cells in areas outside of segmental FN were predominantly CD163+ and CD68+ (scores=1.6 [0.9] and 2.1 [0.8], respectively, versus 0.2 [0.5] for CD3+ cells and 0.2 [0.6] for PMNs; P<0.001 for each). Pericapsular cells were also predominantly CD163+ and CD68+ (scores=2.4 [0.7] and 2.1 [0.7], respectively, versus 1.1 [0.8] for CD3+ cells; P<0.001 for each comparison). Pericapsular PMNs were rare (score=0.1). Endocapillary, extracapillary, and pericapsular CD56+ and CD20+ cells were rarely present in these glomeruli. There were no significant differences in the inflammatory cell phenotypes in biopsies from patients who were anti-MPO–positive (n=8) compared with anti-PR3–positive (n=6).
Infiltrating Cells at Sites of FN Compared with CC in PNGN.
Thirty-two glomeruli studied on immunoperoxidase sections had CCs. Five glomeruli had both CCs and FN (Figure 2). Extracapillary CD163+ cells and PMNs were more numerous in glomeruli with CCs than those with segmental FN (mean score=2.5 [0.8] versus 1.6 [0.9] for CD163+ cells; P<0.001; and 0.9 [0.9] versus 0.2 [0.6] for PMNs; P<0.01) (Figure 2). More CD68+, CD163+, and CD3+ cells were evident at sites of FN in CCs than sites of FN only; however, the numbers of glomeruli available with global CCs and FN were few (n=5). In general, the numbers of CD68+, CD163+, and CD3+ cells and PMNs were increased in all compartments of glomeruli with CCs compared with those with FN only (Figure 2).
Infiltrating Cells at Sites of FN in PNGN Compared with Anti-GBM GN and Immune Complex–Mediated GN.
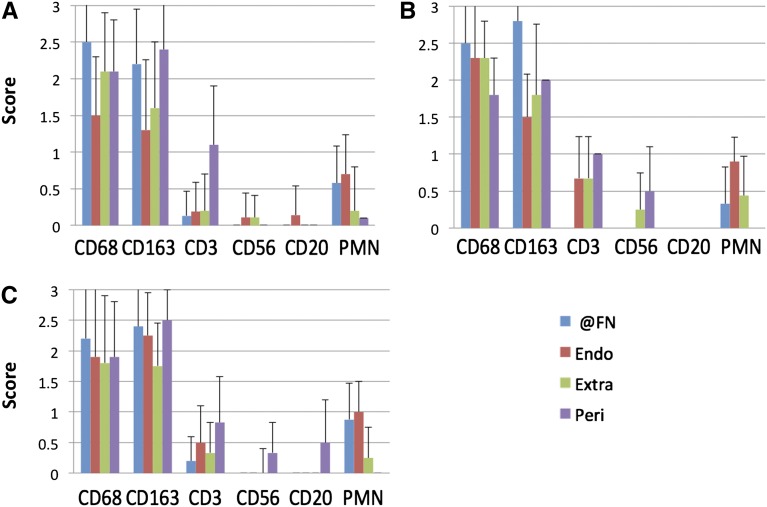
CD163+CD68+ macrophages predominated at sites of segmental FN in PNGN, anti-GBM GN, and immune complex–mediated GN, greatly exceeding the quantity of neutrophils and T cells (score of CD163=2.8 [0.5] and score of CD68=2.5 [0.6] versus score of PMNs=0.3 [0.5] and score of CD3=0 [0] in anti-GBM GN; P<0.001 for each; score of CD163=2.4 [0.7] and score of CD68=2.2 [1.1] versus score of PMNs=0.9 [0.6] and score of CD3=0.2 [0.4] in immune complex–mediated GN; P<0.001 for each) (Figure 3). B and natural killer cells were rarely detected. There were no significant differences in the distribution or frequency of infiltrating phenotypes between these types of necrotizing GN.
Figure 3.
Macrophages are the predominant infiltrates in segmental fibrinoid necrosis of all causes. Infiltrating cell populations in glomeruli with segmental FN from biopsies with (A) pauci-immune necrotizing GN compared with (B) antiglomerular basement membrane GN (10 glomeruli from five patients) and (C) immune complex–mediated GN (nine glomeruli from six patients). The distribution and phenotypes are similar in each group, suggesting common effectors of glomerular injury in FN (data presented as means+SDs). Endo, endocapillary; Extra, extracapillary; @FN, at the site of fibrinoid necrosis; Peri, pericapsular; PMN, polymorphonuclear neutrophil.
Infiltrating Cells in Normal-Appearing Glomeruli from Biopsies with PNGN.
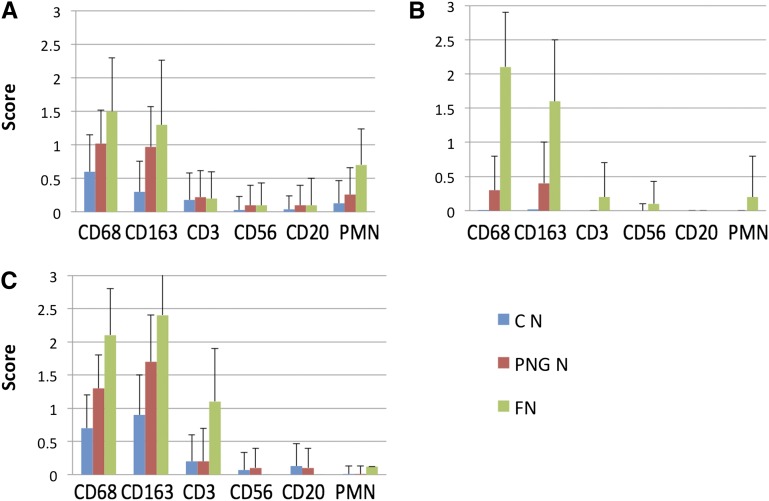
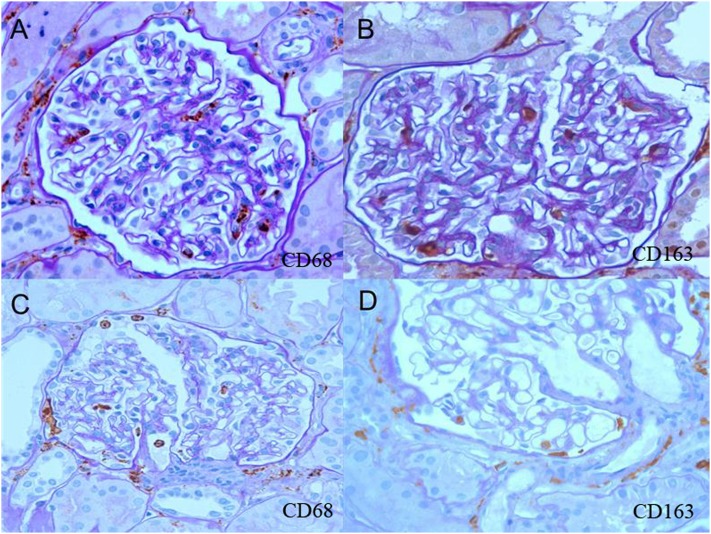
Glomeruli without necrosis or crescents had more CD68+ and CD163+ cells compared with normal controls with thin basement membrane nephropathy in the endocapillary (CD68 scores=1 [0.5] versus 0.6 [0.55]; CD163 scores=1 [0.6] versus 0.3 [0.46]), extracapillary (CD68 scores=0.3 [0.5] versus 0.01 [0.1]; CD163 scores=0.4 [0.57] versus 0.02 [0.13]), and pericapsular (CD68 scores=1.3 [0.5] versus 0.7 [0.5]; CD163 scores=1.7 [0.7] versus 0.9 [0.6]) compartments (P<0.001 for each comparison) as depicted in Figure 4. Biopsies with PNGN had more pericapsular CD3+ cells in normal-appearing glomeruli compared with glomeruli from thin basement membrane nephropathy (score=0.8 [0.4] versus 0.2 [0.5]; P<0.001). Extracapillary CD68+ and CD163+ cells were identified in 31% and 32%, respectively, of normal-appearing glomeruli evaluated from biopsies with PNGN; 75% of biopsies with PNGN had at least one glomerulus with extracapillary CD68+ cells, and 60% of biopsies had extracapillary CD163+ cells in otherwise normal-appearing glomeruli (Figure 5).
Figure 4.
Macrophages are increased in normal-appearing glomeruli in PNGN compared with controls. Infiltrating cells in glomeruli of normal controls with thin basement membrane nephropathy (C N; 63 glomeruli from five patients), normal-appearing glomeruli in pauci-immune necrotizing GN (PNG N; 139 glomeruli from 17 patients), and segmental fibrinoid necrosis in pauci-immune necrotizing GN (FN; 28 glomeruli from 14 patients). There is progressive increase of (A) endocapillary, (B) extracapillary, and (C) pericapsular CD68+ and CD163+ cells with lesser infiltrates of other phenotypes (data presented as means+SDs).
Figure 5.
Macrophage infiltrates in normal-appearing glomeruli from biopsies with PNGN. (A) Endocapillary and pericapsular CD68+ cells. (B) Endocapillary and pericapsular CD163+ cells. (C) Endocapillary and extracapillary CD68+ cells. (D) Endocapillary and extracapillary CD163+ cells.
Interstitial Infiltrates.
To determine the relationship of interstitial infiltrates with the extent of glomerular disease, we divided the index biopsies into three groups by the percentage of normal-appearing glomeruli: >75% (n=6), 60%–75% (n=5), and <60% (n=6). Biopsies with >75% normal-appearing glomeruli had significantly greater interstitial infiltrates of CD68+ (2 [0.3] versus 1 [0.2]) and CD163+ macrophages (2.7 [0.6] versus 1.5 [0.5]) and PMNs (0.65 [0.5] versus 0.08 [0.3]) than controls (P<0.001). Infiltrates of CD3+, CD56+, and CD20+ cells were similar to the controls. There was a slight increase of interstitial CD3+ T cells and PMNs as glomerular involvement by FN and CCs increased (data not shown).
Clinical and Pathologic Correlations
The serum creatinine level at biopsy correlated with the glomerular CD68 score (r=0.74; P=0.001) and glomerular PMN score (r=0.71; P=0.001). The creatinine level also showed weak correlations with the proportion of CCs (r=0.63; P<0.01), the tubulointerstitial inflammatory score (r=0.69; P=0.001), and the tubulointerstitial CD68 score (r=0.59; P<0.01) but not with the extent of segmental FN (r=0.36). There was a negative correlation of the proportion of normal-appearing glomeruli with the serum creatinine level (r=−0.62; P<0.01).
The extent of FN correlated weakly with the glomerular CD68 scores (r=0.51; P=0.05) and had no correlation with other histologic parameters. The extent of CCs correlated with CD68 (r=0.83; P<0.001), CD163 (r=0.8; P<0.001), and PMN (r=0.75; P=0.001) scores in glomeruli and showed weak correlation with glomerular CD3 (r=0.62; P<0.01) and tubulointerstitial CD68 (r=0.59; P<0.01) scores.
Ultrastructural Studies
Ultrastructural lesions in glomeruli from biopsies with PNGN were divided into four groups: (1) exudative (n=15), (2) early (n=8), (3) scarred (n=2), and (4) nonspecific abnormalities (n=12) (Figures 6 and 7). Two patients had no glomeruli, and one patient had severe autolytic artifact.
Figure 6.
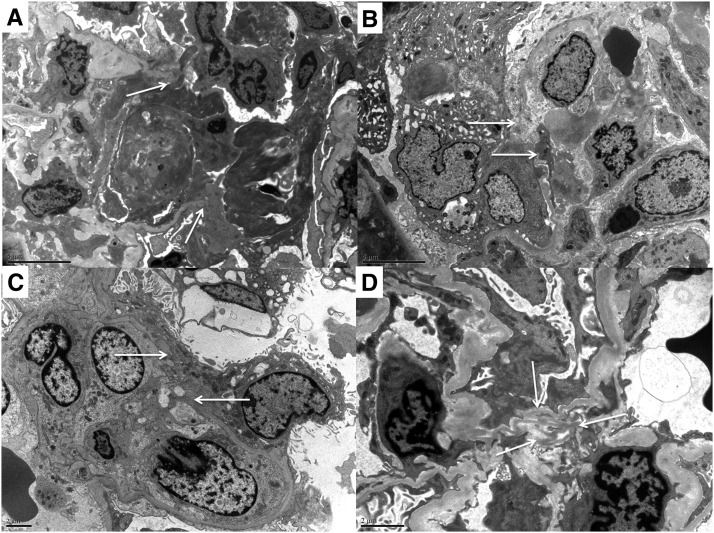
Ultrastructural features of minute exudative fibrinoid necrosis, glomerular basement membrane perforations, and attenuations. (A) Exudative fibrinoid necrosis with glomerular basement membrane (GBM) perforation (arrows), fibrin thrombus attached to endothelium, and fibrin exudate and mononuclear inflammatory cells with long processes attached to fibrin sheaves and thrombus. (B) Early lesion with a minute GBM perforation (arrows), intracapillary mononuclear cells, and swollen epithelioid cells in Bowman’s space. (C) Endocapillary mononuclear cells extending processes through a minute GBM perforation (arrows). (D) Wrinkled redundant GBM at the mesangium (arrows) with cellular processes extending from the capillary lumen (lower middle) and mesangial cells with numerous processes extending toward the redundant GBM.
Figure 7.
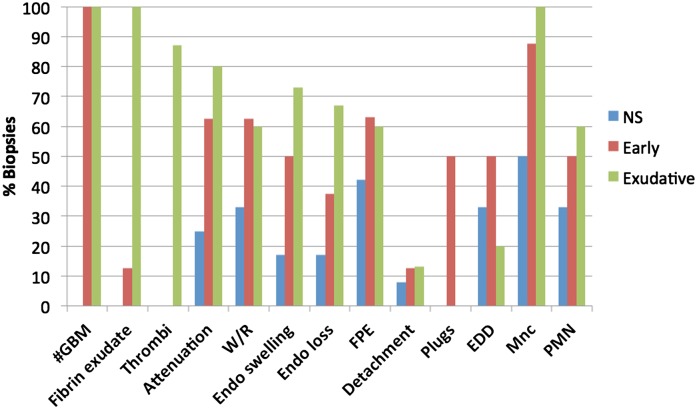
The distribution of ultrastructural lesions seen in biopsies with pauci-immune necrotizing GN (PNGN). Exudative lesions of fibrinoid necrosis (n=15) are shown in green, early lesions (n=8) are shown in red, and glomeruli without apparent diagnostic abnormalities (nonspecific [NS], n=12) are shown in blue. Endothelial injury, glomerular basement membrane attenuation and redundancy, podocyte injury, and mononuclear and polymorphonuclear neutrophil infiltrates are common accompaniments of severe glomerular injury and also present in less injured glomeruli from biopsies with PNGN. Lesions designated NS may, in fact, be the earliest recognizable lesions of PNGN in the correct clinical and pathologic context. EDD, electron dense deposit; FPE, podocyte foot process effacement; #GBM, glomerular basement membrane perforation; Mnc, mononuclear cell; PMN, polymorphonuclear neutrophil; W/R, wrinkling/redundancy.
Exudative lesions of segmental FN were characterized by perforations of the GBM varying from 5 to >25 μm in dimension (Figures 6A and 7), exudation of fibrin and thrombosis, macrophages, and PMNs as depicted in Figure 7. The GBM also had segmental marked attenuation characterized by marked thinning (mean thickness=88 nm, n=11) with redundancy (Figure 6D). Endothelial cells were swollen, detached from the GBM, or lost in capillaries with and without GBM perforation. Podocyte foot processes were effaced segmentally (40%) or globally (20%). Three biopsies had scant segmental electron dense deposits in notch regions (n=1), subendothelial and intramembranous locations (n=1), and mesangial locations (n=1).
The smallest perforations and segments of marked GBM attenuation were classified as early lesions (n=8) (Figure 7). They were identified in the capillary wall (67%) and the mesangium (56%) (Figure 6, B and C). Minute capillary basement membrane perforations measured from <1 to 5 μm. They were accompanied by swelling and detachment of the podocytes and endothelium, indicating an acute injury process. Podocyte cytoplasmic projections plugged minute gaps in the GBM in four of these biopsies. Podocyte bridges were not seen. Monocytes or macrophages were the predominant infiltrating cell type, and cell processes could be seen in contact with the attenuations and at gap sites (Figure 6, B and C). Three of these biopsies had scant electron dense deposits, including subepithelial humps (n=2) and subendothelial or intramembranous deposits (n=2).
Two biopsies had segmental collagen deposition at sites of minute GBM perforation and were classified as scarred lesions. Macrophages were present at these sites. Twelve biopsies had segmental attenuation (25%) and wrinkling with redundancy (33%) of the GBM, one or more infiltrating macrophages (50%) and PMNs (33%), and effacement of the podocyte foot processes (segmental=17% and global=25%) (Figure 7). Podocyte bridges were not observed. Occasional electron dense deposits were evident in four biopsies, including subepithelial humps in the notch region (n=2), subendothelial deposits (n=2), and mesangial deposits (n=1).
Discussion
Involvement of macrophages in AAV has largely been recognized as granuloma formation in disorders characterized by granulomatosis and polyangiitis. In this study, we present novel observations of macrophage involvement in the early phases of necrotizing GN in patients with AAV with and without granulomatosis. CD163+CD68+ macrophages were identified at sites of FN, and macrophages were localized to the minutest sites of GBM injury. Macrophages have long been recognized in CGN (8,11,12,17–19). However, the data supporting a role for macrophages in the pathogenesis of human CGN have been derived from biopsies with diffuse, well developed CCs (15,17–19) with or without rupture of Bowman’s capsular basement membrane (20) and hence, an advanced state of the injury process. The distinction of effectors from responders is difficult in such complex lesions, and therefore, we studied biopsies with predominantly normal-appearing glomeruli. Our findings show that CD163+ macrophages (1) are present in glomerular capillaries in the absence of FN and potentially before these lesions develop, (2) are capable of exudation into Bowman’s space in the absence of FN, (3) accumulate in greater numbers in glomeruli with segmental FN, and (4) are present diffusely in the interstitium in biopsies with limited focal vasculitic GN. The selective accumulation and exudation of CD163+ macrophages may be precursor steps in the evolution of FN and subsequent CC formation. Glomerular endocapillary and interstitial PMN and pericapsular CD3+ T cells were present in greater numbers when lesions of segmental FN developed.
Ultrastructural study of minute segmental glomerular capillary wall perforations revealed attenuation and redundancy of the GBM, acute endothelial and podocyte injury, and colocalization of macrophages and occasional PMNs. Because these lesions were seen frequently in AAV, it seems probable that they precede full-blown exudative FN. GBM perforations have been described in association with endothelial injury, thrombosis, and fibrin exudation (21–23) in older ultrastructural studies of CGN. One study described dissolution or lysis of the capillaries without necrosis and filling of GBM gaps by podocytes and endothelial and mesangial cells (23). In the setting of AAV, these nonspecific features of acute glomerular injury can be seen together in glomeruli with and without FN and thus, considered evidence of early vasculitic injury. Podocyte bridges (24) were not observed.
ANCA can bind to MPO and PR3 in monocytes and macrophages, with resulting production of reactive oxygen radicals and increased adhesion to endothelium (25). Macrophages have an abundance of lysosomal enzymes, with potential for cellular and matrix injury; however, a role for them in vasculitic injury has not been established. It is notable that FN in anti-GBM GN and immune complex–mediated GN had similar distributions of CD163+ macrophages and other inflammatory phenotypes, comparable with lesions in PNGN. A precedent for common infiltrating cell types, independent of the initiating immune mechanism of glomerular injury, has been observed in murine models of anti-GBM GN (26).
Macrophages have been divided into classically activated M1 types, which promote tissue injury, and alternatively activated M2 types, which promote tissue repair (27–30). CD163 has complex biologic functions but is best characterized as a hemoglobin scavenger receptor and an innate immune sensor (27). CD163 expression is recognized as a marker of alternative (M2-type) macrophage differentiation (28,29,31). Calprotectin is a marker of M1-type macrophage differentiation and has been identified in macrophages in CGN (12,32). Studies of M1 macrophages available in the literature (12,32) and the identification of M2 macrophages in our study together seem to indicate that both M1 and M2 macrophage phenotypes may be present simultaneously in active lesions, although we could not confirm the presence of M1-type cells. Whether these are the same macrophages expressing each of these markers, representing plasticity of macrophage differentiation (30) or separate populations of these cells, is uncertain.
The serum creatinine values at the time of the index biopsy correlated with the extent of glomerular macrophage and PMN infiltration but not the extent of FN. These observations are consistent with a functionally significant glomerular inflammatory infiltrate; however, our sample size is rather small. Others have observed correlation of glomerular and interstitial macrophages with serum creatinine at biopsy (19). The extent of glomerular necrosis and crescent formation correlated with the serum creatinine at biopsy in a separate study (33).
PMNs were scarce in normal-appearing glomeruli, more numerous in glomeruli with FN, and still more numerous in glomeruli with CCs. PMN infiltrates also tended to localize at sites of FN. We used only light microscopy and electron microscopy to identify PMN, and therefore, it is likely that the extent of PMN infiltration is underestimated in this study. CD3+ T cells were present predominantly in pericapsular locations in normal-appearing glomeruli and those with FN. Endocapillary, extracapillary, and pericapsular CD3+ T cells increased considerably in glomeruli with CCs. Prior studies of well developed crescentic lesions (11,13,18,19,34) described greater numbers of CD68+ cells, CD3+ cells, and PMNs than were observed in the early lesions of this study. Our observation suggests that T cells may infiltrate the injured glomeruli later than macrophages and are most prominent when CCs are well developed. CD56+ natural killer and CD20+ B cells were notable for their absence from sites of glomerular FN.
In summary, the study findings indicate selective accumulation of CD163+ macrophages in glomeruli with the earliest recognizable lesions of PNGN. CD163+ macrophages accumulated in glomerular capillaries and Bowman’s space in normal-appearing glomeruli and the interstitium, and were more numerous in glomeruli with FN and those with CCs. These observations indicate that alternatively activated macrophages predominate at early lesions of pauci-immune vasculitic GN and suggest a potential effector role for these macrophages in glomerular injury in AAV.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “ANCAs Are Also Antimonocyte Cytoplasmic Autoantibodies,” on pages 4–6.
References
- 1.Jennette JC, Thomas DB: Pauci-immune and antineutrophil cytoplasmic autoantibody-mediated crescentic glomerulonephritis and vasculitis. In: Heptinstall's Pathology of the Kidney, 6th Ed., edited by Jennette JC, Olson JL, Schwartz MM, Silva FG, Philadelphia, Lippincott Williams & Wilkins, 2007, pp 643–673 [Google Scholar]
- 2.Franssen CF, Huitema MG, Muller Kobold AC, Oost-Kort WW, Limburg PC, Tiebosch A, Stegeman CA, Kallenberg CG, Tervaert JW: In vitro neutrophil activation by antibodies to proteinase 3 and myeloperoxidase from patients with crescentic glomerulonephritis. J Am Soc Nephrol 10: 1506–1515, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Kettritz R, Jennette JC, Falk RJ: Crosslinking of ANCA-antigens stimulates superoxide release by human neutrophils. J Am Soc Nephrol 8: 386–394, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Muller Kobold AC, van der Geld YM, Limburg PC, Tervaert JW, Kallenberg CG: Pathophysiology of ANCA-associated glomerulonephritis. Nephrol Dial Transplant 14: 1366–1375, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Falk RJ, Jennette JC: ANCA disease: Where is this field heading? J Am Soc Nephrol 21: 745–752, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Jennette JC, Falk RJ, Gasim AH: Pathogenesis of antineutrophil cytoplasmic autoantibody vasculitis. Curr Opin Nephrol Hypertens 20: 263–270, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kambham N: Crescentic glomerulonephritis: An update on pauci-immune and anti-GBM diseases. Adv Anat Pathol 19: 111–124, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Tipping PG, Holdsworth SR: T cells in crescentic glomerulonephritis. J Am Soc Nephrol 17: 1253–1263, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Ruth A-J, Kitching AR, Kwan RYQ, Odobasic D, Ooi JDK, Timoshanko JR, Hickey MJ, Holdsworth SR: Anti-neutrophil cytoplasmic antibodies and effector CD4+ cells play nonredundant roles in anti-myeloperoxidase crescentic glomerulonephritis. J Am Soc Nephrol 17: 1940–1949, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Yoshioka K, Takemura T, Akano N, Miyamoto H, Iseki T, Maki S: Cellular and non-cellular compositions of crescents in human glomerulonephritis. Kidney Int 32: 284–291, 1987 [DOI] [PubMed] [Google Scholar]
- 11.Cunningham MA, Huang XR, Dowling JP, Tipping PG, Holdsworth SR: Prominence of cell-mediated immunity effectors in “pauci-immune” glomerulonephritis. J Am Soc Nephrol 10: 499–506, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Rastaldi MP, Ferrario F, Crippa A, Dell’Antonio G, Casartelli D, Grillo C, D’Amico G: Glomerular monocyte-macrophage features in ANCA-positive renal vasculitis and cryoglobulinemic nephritis. J Am Soc Nephrol 11: 2036–2043, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Weidner S, Carl M, Riess R, Rupprecht HD: Histologic analysis of renal leukocyte infiltration in antineutrophil cytoplasmic antibody-associated vasculitis: Importance of monocyte and neutrophil infiltration in tissue damage. Arthritis Rheum 50: 3651–3657, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Couser WG: Basic and translational concepts of immune-mediated glomerular diseases. J Am Soc Nephrol 23: 381–399, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, Cosio F, David DSR, Drachenberg C, Einecke G, Fogo AB, Gibson IW, Glotz D, Iskandar SS, Kraus E, Lerut E, Mannon RB, Mihatsch M, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Roberts I, Seron D, Smith RN, Valente M: Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplant 8: 753–760, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Berden AE, Ferrario F, Hagen EC, Jayne DR, Jennette JC, Joh K, Neumann I, Noël L-H, Pusey CD, Waldherr R, Bruijn JA, Bajema IM: Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol 21: 1628–1636, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Hancock WW, Atkins RC: Cellular composition of crescents in human rapidly progressive glomerulonephritis identified using monoclonal antibodies. Am J Nephrol 4: 177–181, 1984 [DOI] [PubMed] [Google Scholar]
- 18.Bolton WK, Innes DJ, Jr., Sturgill BC, Kaiser DL: T-cells and macrophages in rapidly progressive glomerulonephritis: Clinicopathologic correlations. Kidney Int 32: 869–876, 1987 [DOI] [PubMed] [Google Scholar]
- 19.Aasarød K, Bostad L, Hammerstrøm J, Jørstad S, Iversen BM: Wegener’s granulomatosis: Inflammatory cells and markers of repair and fibrosis in renal biopsies—a clinicopathological study. Scand J Urol Nephrol 35: 401–410, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Boucher A, Droz D, Adafer E, Noël LH: Relationship between the integrity of Bowman’s capsule and the composition of cellular crescents in human crescentic glomerulonephritis. Lab Invest 56: 526–533, 1987 [PubMed] [Google Scholar]
- 21.Morita T, Suzuki Y, Churg J: Structure and development of the glomerular crescent. Am J Pathol 72: 349–368, 1973 [PMC free article] [PubMed] [Google Scholar]
- 22.Bohman SO, Olsen S, Petersen VP: Glomerular ultrastructure in extracapillary glomerulonephritis. Acta Pathol Microbiol Scand Suppl 249[Suppl]: 29–54, 1974 [PubMed] [Google Scholar]
- 23.Stejskal J, Pirani CL, Okada M, Mandelanakis N, Pollak VE: Discontinuities (gaps) of the glomerular capillary wall and basement membrane in renal diseases. Lab Invest 28: 149–169, 1973 [PubMed] [Google Scholar]
- 24.Le Hir M, Keller C, Eschmann V, Hähnel B, Hosser H, Kriz W: Podocyte bridges between the tuft and Bowman’s capsule: An early event in experimental crescentic glomerulonephritis. J Am Soc Nephrol 12: 2060–2071, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Nowack R, Schwalbe K, Flores-Suárez LF, Yard B, van der Woude FJ: Upregulation of CD14 and CD18 on monocytes in vitro by antineutrophil cytoplasmic autoantibodies. J Am Soc Nephrol 11: 1639–1646, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Le Hir M: Histopathology of humorally mediated anti-glomerular basement membrane (GBM) glomerulonephritis in mice. Nephrol Dial Transplant 19: 1875–1880, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Ritter M, Buechler C, Langmann T, Orso E, Klucken J, Schmitz G: The scavenger receptor CD163: Regulation, promoter structure and genomic organization. Pathobiology 67: 257–261, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Akila P, Prashant V, Suma MN, Prashant SN, Chaitra TR: CD163 and its expanding functional repertoire. Clin Chim Acta 413: 669–674, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Etzerodt A, Moestrup SK: CD163 and inflammation: Biological, diagnostic, and therapeutic aspects. Antioxid Redox Signal 18: 2352–2363, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barros MHM, Hauck F, Dreyer JH,Kempkes B, Niedobitek G: Macrophage polarisation: An immunohistochemical approach for identifying M1 and M2 macrophages. PLoS ONE 8: e80908, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erwig L-P, Kluth DC, Rees AJ: Macrophage heterogeneity in renal inflammation. Nephrol Dial Transplant 18: 1962–1965, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Pepper RJ, Hamour S, Chavele K-M, Todd SK, Rasmussen N, Flint S, Lyons PA, Smith KGC, Pusey CD, Cook HT, Salama AD: Leukocyte and serum S100A8/S100A9 expression reflects disease activity in ANCA-associated vasculitis and glomerulonephritis. Kidney Int 83: 1150–1158, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furlong TJ, Ibels LS, Eckstein RP: The clinical spectrum of necrotizing glomerulonephritis. Medicine (Baltimore) 66: 192–201, 1987 [DOI] [PubMed] [Google Scholar]
- 34.Rastaldi MP, Ferrario F, Tunesi S, Yang L, D’Amico G: Intraglomerular and interstitial leukocyte infiltration, adhesion molecules, and interleukin-1 alpha expression in 15 cases of antineutrophil cytoplasmic autoantibody-associated renal vasculitis. Am J Kidney Dis 27: 48–57, 1996 [DOI] [PubMed] [Google Scholar]