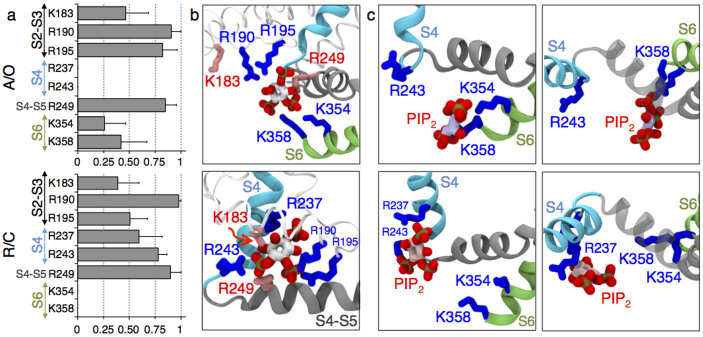
Figure 2. There are two modes of protein-lipid interactions each favored either in the activated/open (A/O, top panels) or resting/closed (R/C, bottom panels) states of Kv7.1.
(a) Probabilities of salt bridges formation between PIP2 and positive residues of Kv7.1 among all subunits. Multiple MD runs are considered. The standards deviations (SDs) are represented as bars. (b) Coordination of PIP2 by residues composing the intrasubunit binding site: K183, R190 and R195 of the S2–S3 loop, R237 and R243 of S4, R249 of the S4–S5 linker, K354 and K358 of the S6 terminus. K183 and R249, the gain-of-function residues, are highlighted in red. (c) Side (left panel) and top (right panel) views of the representative equilibrium position of PIP2 at the intrasubunit binding site. Note that PIP2 anchors the S6 terminus in the A/O state of Kv7.1. When the channel is R/C, the lipid is shifted toward the VSD to interact with the bottom of S4.